可见光介导的二甲基亚砜对n -杂芳烃的亚砜烷基化反应
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
亚砜部分是天然产物和具有药理活性的化合物中普遍存在的特殊结构基序。因此,使直接亚砜基团结合的方法在当代药物发现中具有重要价值。在这项工作中,我们揭示了一个可见光驱动的n -杂芳烃直接亚砜烷基化的光氧化还原迷你型反应。这种转化在异常温和的条件下进行,同时保持良好的官能团耐受性,从而为药物化学应用的后期多样化提供了相当大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
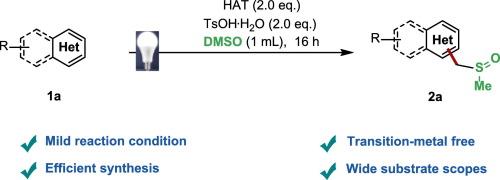
Visible light-mediated CH sulfinylalkylation of N-heteroaromatics via dimethyl sulfoxide
The sulfoxide moiety represents a privileged structural motif prevalent in natural products and pharmacologically active compounds. Consequently, methodologies enabling direct sulfoxide group incorporation hold significant value in contemporary drug discovery. In this work, we disclose a visible-light-driven photoredox Minisci-type reaction for the direct sulfinylalkylation of N-heteroarenes. This transformation proceeds under exceptionally mild conditions while maintaining excellent functional group tolerance, thereby offering considerable potential for late-stage diversification in medicinal chemistry applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


