氨基乙醛二甲基缩醛与α-氧二硫酯在酸性介质中的环化:一种获取2-酰基噻唑的简便方法
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这封信中,我们揭示了在DMF中,在80℃下,氨基乙醛二甲基缩醛与α-氧二硫酯在对甲苯磺酸存在下的环缩合反应生成2-酰基噻唑。该方法减少了α-氧二硫酯合成2-酰基噻唑的步骤,克服了以往α-氧二硫酰胺与溴乙醛缩二乙醛反应的局限性。在各种电中性基团、供电子基团和吸电子基团存在的情况下,证明了反应的普遍性。高总产率和相对较短的反应时间的一些反应是该方案的优点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
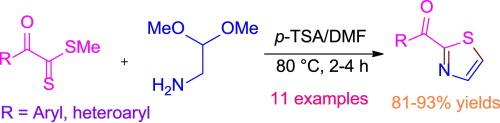
Cyclization of aminoacetaldehyde dimethyl acetal with α-oxodithioesters in acidic media: an easy way to access 2-acylthiazoles
In this letter, we disclose the formation of 2-acylthiazoles by the cyclocondensation of aminoacetaldehyde dimethyl acetal with α-oxodithioesters in the presence of p-tolunesulfonic acid in DMF at 80 °C. The present method reduces a step for the synthesis of 2-acylthiazoles from α-oxodithioesters and thus overcome the limitation of our earlier work which involves the reaction between α-oxothioamides and bromoacetaldehyde diethyl acetal. The generality of the reaction has been demonstrated in the presence of various electro neutral, electron donating and electron withdrawing groups. High overall yields and relatively shorter reaction times for some reactions are the prons of this protocol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


