大气压表面电离质谱法测定氧化的SUS304和镍铬:铬酸(H2CrnO3n+1)作为中间前驱体,形成六价氧化铬负离子HCrnO3n+1−和质子化的氧化物表面作为固体酸
IF 1.7
3区 化学
Q3 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
摘要
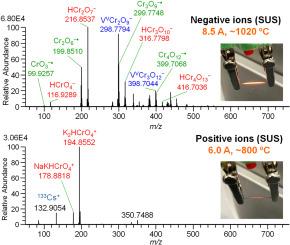
采用常压表面电离质谱法测定了SUS304和镍铬丝加热氧化后产生的负离子和正离子。两种细丝均产生六价氧化铬负离子HCrnO3n+1−(n = 1 - 4)和CrnO3n−•(n = 1 - 4)和正离子A2HCrnO3n+1+ (n = 1 - 3) (A: Na和/或K)。负离子在温度高于~ 1000°C时被检测到,而正离子在温度低于~ 800°C时被检测到。负离子前体假定为铬酸,由反应形成,CrnO3n+ H2O→H2CrnO3n+1,然后电解解离,H2CrnO3n+1→H+ + HCrnO3n+1−发生在固体表面。质子H+可立即被表面氧化物捕获,形成质子化的金属氧化物,该金属氧化物在气相分析物的质子化过程中充当固体Brønsted酸。从加热的铬合金中形成六价氧化铬离子可能对健康有害,因为由于其强氧化性,它们在低浓度下具有致癌性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Atmospheric pressure surface ionization mass spectrometry for oxidized SUS304 and nichrome: chromic acids (H2CrnO3n+1) as intermediate precursors for the formation of hexavalent chromium oxide negative ions HCrnO3n+1− and protonated oxide surface as solid acid
Negative and positive ions generated from heated oxidized SUS304 and nichrome filaments were measured by atmospheric pressure surface ionization mass spectrometry. Both filaments produced hexavalent chromium-oxide negative ions of HCrnO3n+1− (n = 1–4) and CrnO3n−• (n = 1–4) and positive ions of A2HCrnO3n+1+ (n = 1–3) (A: Na and/or K). While negative ions were detected at temperatures above ∼1000 °C, positive ions were detected at much lower temperature above ∼800 °C. The precursors of negative ions were assumed to be chromic acid formed by the reaction, CrnO3n + H2O → H2CrnO3n+1 followed by electrolytic dissociation, H2CrnO3n+1 → H+ + HCrnO3n+1− taking place on the solid surface. The proton H+ may be captured by the surface oxide instantly to form protonated metal oxide which acts a solid Brønsted acid for the protonation of gas-phase analytes. The formation of hexavalent chromium oxide ions from heated chromium alloys may be hazardous to health because they are carcinogenic at low concentrations due to their strong oxidizing properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.60
自引率
5.60%
发文量
145
审稿时长
71 days
期刊介绍:
The journal invites papers that advance the field of mass spectrometry by exploring fundamental aspects of ion processes using both the experimental and theoretical approaches, developing new instrumentation and experimental strategies for chemical analysis using mass spectrometry, developing new computational strategies for data interpretation and integration, reporting new applications of mass spectrometry and hyphenated techniques in biology, chemistry, geology, and physics.
Papers, in which standard mass spectrometry techniques are used for analysis will not be considered.
IJMS publishes full-length articles, short communications, reviews, and feature articles including young scientist features.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


