光催化co释放喷雾水凝胶用于肿瘤术后原位治疗
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
乳腺癌的高侵袭性和转移性增加了术后复发的风险。为了解决这一问题,一种基于海藻酸钠(SA)的复合水凝胶给药系统被开发出来,用于在肿瘤切除部位原位释放一氧化碳(CO)。该系统旨在提高化疗的有效性,提高术后残留肿瘤细胞的清除率,从而防止肿瘤复发和转移。设计了一种金纳米粒子修饰的g-C3N4纳米光催化剂(C3N4/Au),在可见光照射下将肿瘤内的CO2转化为CO。将C3N4/Au装入SA水凝胶中,并以喷雾形式应用于术后切口。SA和Ca2+之间的快速交联反应形成网络结构,从而实现精确的给药。CO在光刺激下在术后肿瘤组织中原位生成,并与叶酸(FA)修饰的阿霉素(DOX)胶束(FA@DM)结合,实现CO治疗与化疗的有效协同。复合水凝胶给药系统不仅增加了肿瘤内CO的浓度,降低了全身毒性,而且通过增加肿瘤细胞内的氧化应激,提高了化疗的有效性。为高效、精准的术后肿瘤治疗提供了一种新的安全策略,降低了肿瘤复发和转移的风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
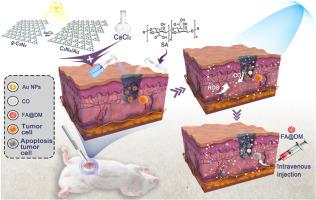
Photocatalytic CO-releasing spray hydrogel for in situ postoperative cancer treatment
The high invasiveness and metastatic potential of breast cancer increase the risk of postoperative recurrence. To address this issue, a composite hydrogel drug delivery system based on sodium alginate (SA) has been developed for the in situ release of carbon monoxide (CO) at the tumor resection site. This system aims to enhance the effectiveness of chemotherapy and improve the clearance of residual tumor cells after surgery, thereby preventing tumor recurrence and metastasis. A gold nanoparticle-modified g-C3N4 nanophotocatalyst (C3N4/Au) has been designed to convert CO2 within the tumor into CO under visible light irradiation. The C3N4/Au is loaded into an SA hydrogel and applied to the postoperative incision in a spray form. The rapid crosslinking reaction between SA and Ca2+ forms a network structure, enabling precise drug delivery. CO is generated in situ in the postoperative tumor tissue under light stimulation and is combined with folic acid (FA) modified doxorubicin (DOX) micelles (FA@DM) to achieve effective synergy between CO therapy and chemotherapy. The composite hydrogel drug delivery system not only increases the concentration of CO within the tumor and reduces systemic toxicity but also enhances the effectiveness of chemotherapy by increasing oxidative stress within tumor cells. It provides a new and safe strategy for efficient and precise postoperative tumor treatment, reducing the risk of tumor recurrence and metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


