在Pd(II)催化下,通过瞬时定向基团激活的C-H烯化反应获得平面手性二茂铁
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
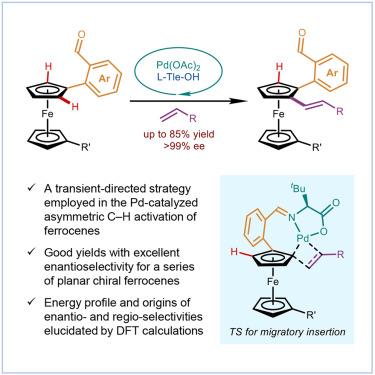
钯催化的不对称碳氢功能化是合成平面手性二茂铁的有利方法。此类别下的先前示例实际上依赖于预安装的强定向组。瞬态定向策略近年来取得了相当大的成功。然而,平面手性二茂铁的合成实例报道有限。在此,我们报道了在Pd催化下,以l-叔亮氨酸作为瞬态导向助剂,与一系列缺电子烯烃进行了二茂铁的不对称C-H烯化反应。该反应显示出广泛的底物范围,目标平面手性二茂铁的收率高(高达85%),对映体选择性高(高达99% ee)。综合机理研究表明,在C-H活化后,环应变在七元palladacycle中的储存是我们的反应设计成功的关键。它保证了迁移插入作为一个动力学上可行的步骤,以释放菌株的方式发生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Accessing planar chiral ferrocenes via transient directing group-enabled C–H alkenylation under Pd(II) catalysis
Pd-catalyzed asymmetric C–H functionalization is a privileged method for the synthesis of planar chiral ferrocenes. Previous examples under this category virtually rely on a preinstalled strong directing group. The transient directing strategy has witnessed considerable success in recent years. However, only limited examples have been reported for the synthesis of planar chiral ferrocenes. Herein, we report an asymmetric C–H alkenylation of ferrocenes with an array of electron-deficient olefins under Pd catalysis with l-tert-leucine as the transient-directing auxiliary. This reaction exhibits a wide substrate scope, and the target planar chiral ferrocenes are prepared in good yields (up to 85%) with exceptional enantioselectivity (up to >99% ee). Comprehensive mechanistic studies suggest that the storage of ring strain in a seven-membered palladacycle after C–H activation is the key to the success of our reaction design. It guarantees the migratory insertion as a kinetically feasible step that occurs in a strain-releasing manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


