基于dna的纳米结构用于细胞膜受体调控和疾病治疗
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
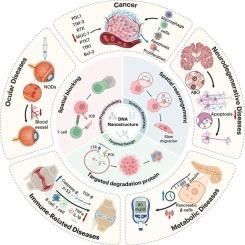
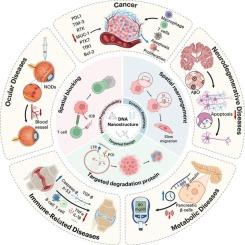
细胞膜受体的异常表达和功能障碍与各种重大疾病的发生和发展密切相关,如癌症、神经退行性疾病、炎症等。然而,传统的膜蛋白调控策略,如小分子抑制剂或基于抗体的治疗,面临着一些挑战,包括靶标依赖性,有限的降解范围,以及耐药性的发展。近年来,DNA纳米结构由于其高可编程性、精确的空间控制和动态响应性,成为精确调节膜受体的创新解决方案。本文综述了DNA纳米结构用于膜蛋白调控的设计策略和最新进展,重点介绍了它们在膜受体的空间阻断、空间重组和靶向降解中的关键作用。通过合理设计DNA折纸、基于适配体的纳米阵列和动态响应装置,研究人员已经实现了对受体二聚化、寡聚化和膜区隔化的精确控制,从而调节下游信号通路。此外,基于蛋白水解靶向嵌合体(PROTACs)、溶酶体靶向嵌合体(LYTACs)和自噬-溶酶体途径的DNA纳米降解平台显著提高了膜蛋白降解效率,同时表现出优异的肿瘤选择性。DNA纳米结构已经成功地应用于癌症免疫治疗、神经退行性疾病的干预和代谢紊乱的调节,为靶向以前“不可药物”的蛋白质提供了新的策略。本文综述了该领域的最新突破,并概述了DNA纳米结构用于膜蛋白调控的未来方向和临床翻译潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
DNA-based nanostructures for cell membrane receptor regulation and disease treatment
The aberrant expression and dysfunction of cell membrane receptors are closely associated with the onset and progression of various major diseases, such as cancer, neurodegenerative disorders, and inflammation. However, conventional membrane protein regulation strategies, such as small-molecule inhibitors or antibody-based therapies, face several challenges, including target dependency, limited degradation scope, and the development of drug resistance. In recent years, DNA nanostructure has emerged as an innovative solution for the precise modulation of membrane receptors, owing to its high programmability, precise spatial control, and dynamic responsiveness. This review provides a comprehensive overview of the design strategies and recent progress in the application of DNA nanostructures for membrane protein regulation, with a particular emphasis on their pivotal roles in spatial blockade, spatial reorganization, and targeted degradation of membrane receptors. By rationally designing DNA origami, aptamer-based nanoarrays, and dynamic responsive devices, researchers have achieved precise control over receptor dimerization, oligomerization, and membrane compartmentalization, thereby modulating downstream signaling pathways. In addition, DNA nano-degradation platforms based on proteolysis-targeting chimeras (PROTACs), lysosome-targeting chimeras (LYTACs), and the autophagy-lysosome pathway have significantly enhanced the efficiency of membrane protein degradation while demonstrating excellent tumor selectivity. DNA nanostructures have been successfully applied in cancer immunotherapy, interventions for neurodegenerative diseases, and the regulation of metabolic disorders, offering new strategies for targeting previously “undruggable” proteins. This review highlights recent breakthroughs in the field and outlines future directions and clinical translation potential of DNA nanostructures for membrane protein regulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


