生理回路通过维持rr γ - t的表观遗传结构来控制第3组先天淋巴样细胞的可塑性
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
肠道每天都会经历微生物和营养物质的波动,这些波动与调节营养吸收和免疫功能的昼夜节律一致。第3组先天淋巴样细胞(ILC3s)通过白细胞介素-22 (IL-22)支持肠道稳态,但可以转化为产生干扰素-γ的ILC1s。昼夜节律蛋白如何控制这种可塑性尚不清楚。在这里,我们发现昼夜节律蛋白rev - erba和rev - erbb β维持ILC3的身份。它们的联合缺失促进了ilc3到ilc1的转化,降低了能量代谢和IL-22的产生,增加了干扰素γ的产生,并增加了对啮齿柠檬酸杆菌感染的易感性。单细胞多组学和基因编辑发现,rev - erba /REV-ERBβ缺乏上调转录因子NFIL3,通过Rorc基因中- 2 kb的顺式调控元件抑制rorγ - t的表达,使细胞转向t- bet驱动状态。染色质和代谢分析表明,rev - erbb α/ rev - erbb β的缺失重新编程了调节和代谢回路。因此,rev - erbb α/ rev - erbb β通过调节控制rr γt表达的时钟基因来保护肠道完整性,并保持ILC3的特性和对肠道炎症的抵抗力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
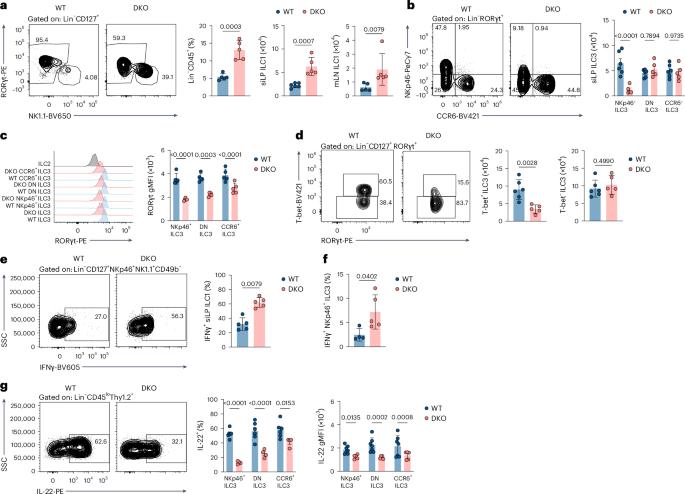
Circadian circuits control plasticity of group 3 innate lymphoid cells by sustaining epigenetic configuration of RORγt
The gut experiences daily fluctuations in microbes and nutrients aligned with circadian rhythms that regulate nutrient absorption and immune function. Group 3 innate lymphoid cells (ILC3s) support gut homeostasis through interleukin-22 (IL-22) but can convert into interferon-γ-producing ILC1s. How circadian proteins control this plasticity remains unclear. Here we showed that the circadian proteins REV-ERBα and REV-ERBβ maintain ILC3 identity. Their combined deletion promoted ILC3-to-ILC1 conversion, reduced energy metabolism and IL-22 production, increased interferon-γ production, and heightened susceptibility to Citrobacter rodentium infection. Single-cell multiomics and gene editing revealed that REV-ERBα/REV-ERBβ deficiency upregulated the transcription factor NFIL3, which repressed the expression of RORγt via a –2-kb cis-regulatory element in the Rorc gene, shifting cells toward a T-bet-driven state. Chromatin and metabolic analyses indicated that REV-ERBα/REV-ERBβ loss reprogrammed regulatory and metabolic circuits. Thus, REV-ERBα/REV-ERBβ safeguard gut integrity by regulating clock genes that control RORγt expression and preserve ILC3 identity and resistance to intestinal inflammation. Colonna and colleagues show that the clock genes encoding REV-ERBα and REV-ERBβ maintain ILC3 functions in the gut by controlling the expression of RORγt.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


