镍催化芳香族碘化物对Isatins的不对称还原加成:手性3-羟基-2-氧吲哚的一般途径。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
一种镍催化的芳基碘化物与isatins的对映选择性还原加成已经被开发出来,这是由一种新设计的foxap型配体实现的,该配体带有远端大块的硅基。该反应以Mn为还原剂,在温和条件下进行,产率高,对映选择性好。该方法具有广泛的底物范围,为合成有价的手性含四元立体中心的3-羟基-2-氧吲哚提供了一种实用有效的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
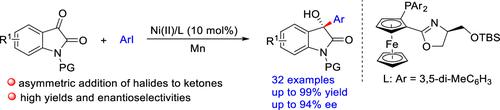
Ni-Catalyzed Asymmetric Reductive Addition of Aromatic Iodides to Isatins: A General Access to Chiral 3-Hydroxy-2-Oxindoles
A nickel-catalyzed enantioselective reductive addition of aryl iodides with isatins has been developed, enabled by a newly designed FOXAP-type ligand bearing a distal bulky silyl group. The reaction proceeds under mild conditions with Mn as the reductant, delivering 3-aryl-3-hydroxyoxindoles in high yields and good to excellent enantioselectivities. This protocol exhibits a broad substrate scope and provides a practical and efficient approach for the synthesis of valuable chiral 3-hydroxy-2-oxindoles bearing a quaternary stereocenter.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


