少突胶质细胞中葡萄糖依赖的嗜胰岛素多肽受体信号增加GLP-1R激动作用的减肥作用
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
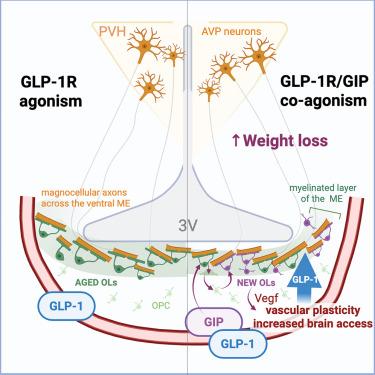
下一代肥胖药物利用葡萄糖依赖性胰岛素性多肽和胰高血糖素样肽1受体(GIPR和GLP-1R)的活性,但其作用机制尚不清楚。在这里,我们报道了GIPR在少突胶质细胞中富集,并且GIPR信号双向调节少突胶质细胞的发生。在少突胶质细胞中GIPR缺失的小鼠中,GIPR激动作用不能增强GLP-1R激动作用的减肥效果。从机制上讲,GIPR激动作用增加了GLP-1R激动剂的脑通路,而这种作用需要少突胶质细胞中的GIPR信号传导。此外,我们发现室旁下丘脑的抗利尿激素神经元对于GLP-1R激活的减肥反应是必要的,通过外周给药GLP-1R激动剂通过其轴突室靶向,并且这种通路通过激活少突胶质细胞中的GIPR而增加。总的来说,我们的研究结果确定了一种新的机制,通过这种机制,肠促胰岛素疗法可能在过度肥胖的管理中促进协同减肥。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Glucose-dependent insulinotropic polypeptide receptor signaling in oligodendrocytes increases the weight-loss action of GLP-1R agonism
The next generation of obesity medicines harness the activity of the glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptors (GIPR and GLP-1R), but their mechanism of action remains unclear. Here, we report that the GIPR is enriched in oligodendrocytes and GIPR signaling bidirectionally regulates oligodendrogenesis. In mice with adult-onset deletion of GIPR in oligodendrocytes, GIPR agonism fails to enhance the weight-loss effects of GLP-1R agonism. Mechanistically, GIPR agonism increases brain access of GLP-1R agonists, and GIPR signaling in oligodendrocytes is required for this effect. In addition, we show that vasopressin neurons of the paraventricular hypothalamus are necessary for the weight-loss response to GLP-1R activation, targeted by peripherally administered GLP-1R agonists via their axonal compartment, and this access is increased by activation of the GIPR in oligodendrocytes. Collectively, our findings identify a novel mechanism by which incretin therapies may function to promote synergistic weight loss in the management of excess adiposity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


