Dapk2功能障碍导致Mic60乳酸化和线粒体代谢重编程,促进肺癌EGFR-TKI耐药和转移
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKI)的耐药通常与肿瘤转移有关,因此控制转移至关重要。在这里,我们确定了一个负责癌症转移和耐药性发展的关键信号中枢:线粒体嵴重塑和代谢重编程,使用anoikis抗性细胞模型和小鼠尾静脉转移模型。与敏感细胞相比,egfr - tki耐药细胞表现出更强的anoikis抗性(AR)和线粒体代谢,使其更容易转移。死亡相关蛋白激酶2 (Dapk2)的功能障碍改变了线粒体嵴中的Mic60蛋白,增加了嵴的丰度和紧密度,激活了线粒体代谢。Mic60蛋白的乳酸化可能是影响线粒体嵴重构和激活线粒体代谢的关键机制。我们的研究结果阐明了线粒体形态动力学和代谢重编程在耐药和转移中的作用和潜在机制,为克服肺癌EGFR-TKI耐药和转移提供了潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
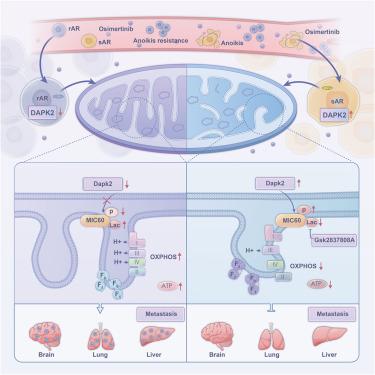
Dapk2 dysfunction leads to Mic60 lactylation and mitochondrial metabolic reprogramming, promoting lung cancer EGFR-TKI resistance and metastasis
The epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) resistance is often linked to tumor metastasis, making control of metastasis crucial. Here, we identified a critical signaling hub responsible for cancer metastasis and resistance development: mitochondrial cristae remodeling and metabolic reprogramming, using an anoikis-resistant cell model and a mouse tail vein metastasis model. EGFR-TKI-resistant cells exhibited stronger anoikis resistance (AR) and mitochondrial metabolism compared with sensitive cells, making them more prone to metastasis. Dysfunction of death-associated protein kinase 2 (Dapk2) altered Mic60 protein in mitochondrial cristae, increasing the abundance and compactness of the cristae and activating mitochondrial metabolism. Lactylation of the Mic60 protein may be the critical mechanism affecting the restructuring of mitochondrial cristae and activating mitochondrial metabolism. Our findings elucidate the role and underlying mechanisms of mitochondrial morphological dynamics and metabolic reprogramming in resistance and metastasis, offering potential therapeutic targets to overcome EGFR-TKI resistance and metastasis in lung cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


