遗传性TRPV4缺失相关肠道菌群减轻糖尿病性心肌病小鼠心功能障碍
IF 4.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
糖尿病性心肌病(DCM)是一种以心功能障碍和心肌纤维化为特征的严重糖尿病并发症。最近的研究强调肠心轴在DCM发展中的重要性。本研究探讨了系统遗传TRPV4基因敲除对DCM进展的影响,并探讨了涉及肠道微生物群调节和肠道屏障完整性的潜在机制。去除DCM小鼠的TRPV4可显著提高心脏功能,减少心肌纤维化,并改变肠道微生物群的组成,导致酸化拟杆菌(Bacteroides acidifaciens, BA)显著增加。TRPV4缺失也上调紧密连接蛋白(Zonula occluden -1 (ZO-1), Occludin和Claudin-1)并降低血清脂多糖水平。此外,将TRPV4基因敲除的DCM供者的粪便微生物群移植到DCM受体中,在小鼠中复制了这些心脏保护作用,给予BA可改善心功能并减轻纤维化。我们的研究表明,TRPV4基因缺失的心脏保护作用与肠道微生物组的变化有关,突出了TRPV4、肠道和心脏之间的联系在DCM的疾病机制和潜在治疗策略中的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
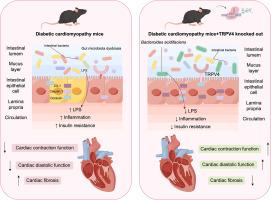
Genetical TRPV4 deletion-associated gut microbiota alleviates cardiac dysfunction in mice with diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is a serious complication associated with diabetes that characterized by the cardiac dysfunction and myocardial fibrosis. Recent studies emphasize the significance of the gut-heart axis in the development of DCM. This current study investigates the effect of systematic-genetical TRPV4 knockout on DCM progression and explores the underlying mechanisms involving gut microbiota modulation and intestinal barrier integrity. The removal of TRPV4 in mice with DCM markedly enhances cardiac performance, decreases myocardial fibrosis, and modifies the composition of gut microbiota, resulting in a significant rise in Bacteroides acidifaciens (BA). TRPV4 deletion also upregulates tight junction proteins (Zonula occludens-1 (ZO-1), Occludin, and Claudin-1) and reduces serum lipopolysaccharide levels. Furthermore, fecal microbiota transplantation from the DCM donors with TRPV4 knockout to the DCM receptors replicates these cardioprotective effects in mice, and administration of BA improves cardiac function and relieves the fibrosis. Our study suggests that the cardioprotective effects of the genetic deletion of TRPV4 are related to changes in the gut microbiome, highlighting the importance of the connection between TRPV4, the gut, and the heart in the disease mechanism and potential therapeutic strategies for DCM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


