ALKBH5通过m6A去甲基化调节COL5A1 mRNA的稳定性,促进烟草致癌物质nnk诱导的肺癌的发生和发展
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
烟草特异性致癌物4-(甲基亚硝胺)-1-(3-吡啶基)-1-丁酮(NNK)因其在肺癌中的致癌作用而被广泛研究。然而,n6 -甲基腺苷(m6A)甲基化在nnk诱导的癌变中的作用尚不清楚。本研究的目的是探讨NNK是否通过失调m6A甲基化促进肺癌发生,重点关注去甲基化酶ALKBH5及其靶点COL5A1。方法分析nnk暴露(100 mg/L, 24 h)的Beas-2B支气管上皮细胞、nnk诱导的恶性转化的Beas-2B (2B-NNK)细胞和配对的肺肿瘤组织中m6A的水平。甲基化RNA免疫沉淀测序、RNA测序和生物信息学鉴定COL5A1为关键靶点。我们进行了COL5A1和ALKBH5敲低的增殖、凋亡、迁移和侵袭实验以及COL5A1稳定性实验。然后我们过表达COL5A1并敲低ALKBH5进行表型拯救实验。结果暴露于nnk的Beas-2B细胞、2B-NNK细胞和人肺肿瘤组织中m6A水平显著降低。COL5A1在2B-NNK细胞中表现出低甲基化和过表达。COL5A1敲低抑制2B-NNK细胞的增殖、迁移和侵袭,同时增加细胞凋亡。m6A去甲基化酶ALKBH5在2B-NNK细胞中上调。它的敲除提高了全球m6A水平,降低了COL5A1 mRNA的稳定性,并逆转了恶性表型。COL5A1过表达也增强了2B-NNK细胞的增殖、迁移和侵袭能力,而同时下调ALKBH5表达可恢复这些增强能力。结论alkbh5通过COL5A1的m6A去甲基化驱动nnk诱导的肺癌,促进肿瘤进展。靶向ALKBH5-COL5A1轴可能提供针对烟草相关癌症的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
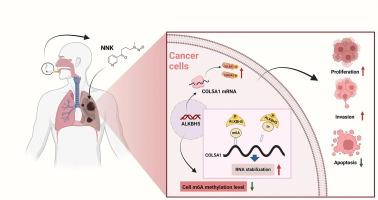
ALKBH5 modulates COL5A1 mRNA stability via m6A demethylation to promote tobacco carcinogen NNK-induced lung cancer occurrence and progression
Introduction
The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), has been extensively studied for its carcinogenic role in lung cancer. However, the involvement of N6-methyladenosine (m6A) methylation in NNK-induced carcinogenesis remains unclear.Objectives
The aim of this study is to investigate whether NNK promotes lung carcinogenesis by dysregulating m6A methylation, focusing on the demethylase ALKBH5 and its target COL5A1.Methods
Levels of m6A were analyzed in NNK-exposed (100 mg/L, 24 h) Beas-2B bronchial epithelial cells, NNK-induced malignant-transformed Beas-2B (2B-NNK) cells, and paired lung tumor tissues. Methylated RNA immunoprecipitation sequencing, RNA sequencing, and bioinformatics identified COL5A1 as a key target. We performed proliferation, apoptosis, migration and invasion experiments involving COL5A1 and ALKBH5 knockdown and COL5A1 stability experiments. We then overexpressed COL5A1 and knocked down ALKBH5 for phenotypic rescue experiments.Results
Levels of m6A were significantly reduced in NNK-exposed Beas-2B cells, 2B-NNK cells, and human lung tumor tissues. COL5A1 exhibited hypomethylation and overexpression in 2B-NNK cells. COL5A1 knockdown suppressed 2B-NNK cell proliferation, migration, and invasion, while increasing apoptosis. The m6A demethylase ALKBH5 was upregulated in 2B-NNK cells. Its knockdown elevated global m6A levels, reduced COL5A1 mRNA stability, and reversed malignant phenotypes. COL5A1 overexpression also enhanced the proliferation, migration, and invasion abilities of 2B-NNK cells, whereas concurrent downregulation of ALKBH5 expression resulted in the restoration of these enhancements.Conclusion
ALKBH5 drives NNK-induced lung cancer via m6A demethylation of COL5A1, promoting tumor progression. Targeting the ALKBH5-COL5A1 axis may offer therapeutic strategies against tobacco-related cancers.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


