甲状旁腺激素受体激动剂治疗骨质疏松症
IF 32.7
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
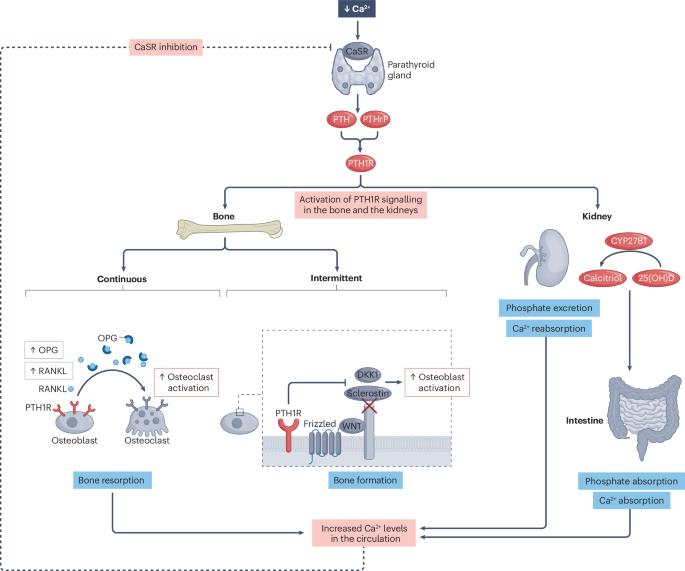
甲状旁腺激素(PTH)调节骨稳态。间歇性暴露于甲状旁腺激素导致骨形成大于骨吸收,这种影响已通过开发甲状旁腺激素和甲状旁腺激素相关蛋白1型受体(PTH1R)激动剂来治疗骨质疏松症。Teriparatide是PTH前34个氨基酸的类似物,abaloparatide在结构上类似于PTH相关蛋白(PTHrP),是目前临床使用的PTH1R激动剂。这两种药物均可增加腰椎、股骨颈和全髋关节的骨密度。特立帕肽或阿巴帕肽的随机对照试验也提供了椎体和非椎体骨折减少的证据。ACTIVE试验表明,阿巴巴拉肽治疗骨质疏松性骨折的疗效略高于特立帕肽(作为探索性终点)。在一项真实世界的观察性研究中,对髋部骨折也有类似的潜在优势。这些药物的副作用通常是短暂的,尽管使用小鼠模型的研究表明存在骨肉瘤的风险,但在广泛的人体研究中没有观察到这种风险。总的来说,特立帕肽和阿巴帕肽都显示出令人信服的临床疗效和成本效益,并具有令人放心的安全性。它们对骨矿物质密度和抗骨折作用的潜在差异为区分提供了途径,但需要在适当设计的研究中进一步验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Parathyroid hormone receptor agonists in the management of osteoporosis
Parathyroid hormone (PTH) regulates bone homeostasis. Intermittent exposure to PTH results in bone formation being greater than bone resorption, and this effect has been harnessed through the development of agonists of the PTH and PTH-related protein type 1 receptor (PTH1R) to treat osteoporosis. Teriparatide, an analogue of the first 34 amino acids of PTH, and abaloparatide, which resembles PTH-related protein (PTHrP) in structure, are PTH1R agonists currently in clinical use. Both medications have been shown to increase bone mineral density at the lumbar spine, femoral neck and total hip. Randomized controlled trials with teriparatide or abaloparatide have also provided evidence of reduction in vertebral and non-vertebral fractures. The ACTIVE trial suggested slightly greater efficacy for major osteoporotic fractures (as an exploratory end point) for abaloparatide than for teriparatide. A similar potential superiority was suggested for hip fracture in a real-world, observational study. Side effects of these medications are usually transient, and although a risk of osteosarcoma was suggested by studies using murine models, no such risk has been observed in extensive human studies. Overall, both teriparatide and abaloparatide have demonstrated convincing clinical effectiveness and cost-effectiveness, with a reassuring safety profile. Potential differences in their effects on bone mineral density and their antifracture effects offer avenues for differentiation but require further validation in appropriately designed studies. This Review summarizes clinical effectiveness, health economics and safety data on the parathyroid hormone receptor agonists teriparatide and abaloparatide, discussing potential strategies and drug combinations to achieve best outcomes in patients with osteoporosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


