塞尼达唑-扁桃酸共晶体系的制备与表征:增强溶出特性、抗菌性能和理论上的药物相似性评估。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
本文介绍了以杏仁酸(MND)为共晶剂,通过缓慢溶剂蒸发法制备了第一个塞克硝唑(SNZ)共晶体系,旨在开发改进的抗菌剂型。通过粉末x射线衍射(PXRD)证实了SNZ-MND体系的共晶性质,结果表明,在180天以上的时间里,SNZ-MND体系的共晶比例为1:1。结构、吸湿性、光谱和热性能进行了深入的研究,并得到了计算计算的支持,包括Hirshfeld表面分析、密度泛函理论(DFT)和计算机药代动力学模拟。在气相和乙醇中的DFT计算提供了SNZ-MND异源二聚体的热力学、几何、电子和振动参数的见解。优化后的结构表明,SNZ的咪唑环和MND的羧基之间的氢键是一个关键的稳定相互作用。热力学数据证实了这种相互作用的自发性,在气相中相互作用能为-2.02 kcal/mol。通过将实验和计算的红外光谱相关联,确定了振动模式。热分析结果表明,该体系的玻璃化转变温度为106.5℃,具有良好的热结构稳定性。在磷酸盐缓冲液(pH 6.8)中的溶解研究表明,SNZ- mnd共晶体系的溶解速率比结晶SNZ高1.22倍,这是影响生物利用度的关键因素。最终平台浓度反映了汇条件下的实验设置。同样,表观溶解度测量表明,与药物的结晶形式相比,溶解浓度增加了22%(39.5±1.2 mg/mL)。重要的是,与分离的化合物相比,SNZ-MND(1:1)共晶对金黄色葡萄球菌、肺炎克雷伯菌和铜绿假单胞菌的抗菌活性增强。这些发现强调了新开发的SNZ-MND共晶体系作为一种有前途的抗菌二元组合物的潜力,该组合物具有改进的物理化学和生物特性,可用于药物剂型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
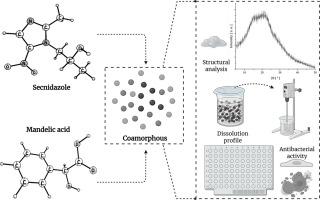
Preparation and characterization of a secnidazole-mandelic acid coamorphous system: enhanced dissolution profile, antibacterial properties, and theoretical druglikeness assessment
This work describes the successful preparation of the first secnidazole (SNZ) coamorphous system using mandelic acid (MND) as a coformer via the slow solvent evaporation method, aiming to develop improved antimicrobial dosage forms. The coamorphous nature of the SNZ-MND system was confirmed by powder X-ray diffraction (PXRD), which revealed that the 1:1 ratio remained stable and amorphous for over 180 days. Its hygroscopicity and the structural, spectroscopic, and thermal properties were thoroughly investigated, supported by computational calculations, including Hirshfeld surface analysis, density functional theory (DFT), and in silico pharmacokinetic simulations. DFT calculations in the gas phase and ethanol provided insights into the thermodynamic, geometric, electronic, and vibrational parameters of the SNZ-MND heterodimer. The optimized geometry indicated that a hydrogen bond between the imidazole ring of SNZ and the carboxylic group of MND is a key stabilizing interaction. Thermodynamic data confirmed the spontaneity of this interaction, with an interaction energy of -2.02 kcal/mol in the gas phase. Vibrational modes were securely assigned by correlating experimental and calculated infrared spectra. Thermoanalytical analysis revealed a glass transition temperature of 106.5 °C, highlighting the system's thermostructural stability. Dissolution studies in phosphate buffer (pH 6.80) showed that the SNZ-MND coamorphous system exhibits a 1.22-fold higher dissolution rate than crystalline SNZ, a critical factor for bioavailability. The final plateau concentrations reflected the experimental setup under sink conditions. Similarly, apparent solubility measurements indicated a 22 % (39.5 ± 1.2 mg/mL) increase in dissolved concentration compared to the crystalline form of the drug. Importantly, the SNZ-MND (1:1) coamorphous system exhibited enhanced antibacterial activity against Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa compared to the isolated compounds. These findings underscore the potential of the newly developed SNZ-MND coamorphous system as a promising antimicrobial binary composition with improved physicochemical and biological properties to be used in pharmaceutical dosage forms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


