酸焙浸法处理AVR工艺铜沉淀
摘要
铜沉淀来自韩国一家金矿厂采用AVR(酸化-挥发-再生)技术的氰化废水处理过程,通常测定值为44.53 wt % Cu, 5.56 wt % Fe and 16.81 wt % S. CuSCN and Cu2Fe(CN)6 are major copper compounds in the precipitate and in the past it has been sold as raw material for pyrometallurgy of copper. Low-temperature (\( \leqq {\kern 1pt} 250^\circ {\text{C}}\)) sulfuric acid baking followed by acid leaching has been evaluated in order to recover copper and the experimental results showed that by acid baking under following condition: 250°C, 60 min and ratio of 98 wt % H2SO4/copper precipitate 2.4, ~98.5 wt % copper was released to the leach solution. During baking Cu2Fe(CN)6 preferentially reacted with sulfuric acid over CuSCN. The leachate containing Cu and Fe can be sent to conventional SX-EW process to produce electrolytic copper. And a little residue (~3.2 wt % of copper precipitate weight) is formed after acid leaching, Cu content in it is 20.8 wt % and Cu2S and CuS are major copper compounds. Possible reactions were proposed for sulfuric acid baking of copper precipitate. HCN formed during baking is reoxidized to CO2 and N2, so recovery of cyanide during baking is of no significance. Based on this investigation, new process for treatment of copper precipitate is proposed for copper recovery. The process mainly consists of acid baking, acid leaching and SX-EW. And treatment of off-gas is needed in view of environmental issue.
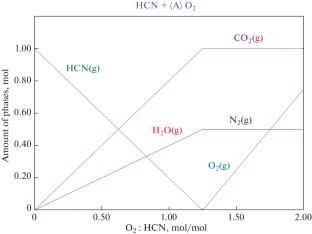
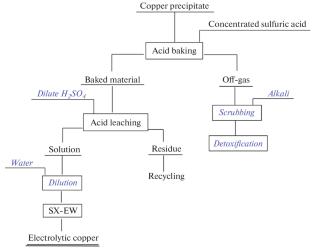
Copper precipitate comes from cyanidation wastewater treatment process using AVR (acidification-volatilization-regeneration) technology in a Korean gold plant and typically assays 44.53 wt % Cu, 5.56 wt % Fe and 16.81 wt % S. CuSCN and Cu2Fe(CN)6 are major copper compounds in the precipitate and in the past it has been sold as raw material for pyrometallurgy of copper. Low-temperature (\( \leqq {\kern 1pt} 250^\circ {\text{C}}\)) sulfuric acid baking followed by acid leaching has been evaluated in order to recover copper and the experimental results showed that by acid baking under following condition: 250°C, 60 min and ratio of 98 wt % H2SO4/copper precipitate 2.4, ~98.5 wt % copper was released to the leach solution. During baking Cu2Fe(CN)6 preferentially reacted with sulfuric acid over CuSCN. The leachate containing Cu and Fe can be sent to conventional SX-EW process to produce electrolytic copper. And a little residue (~3.2 wt % of copper precipitate weight) is formed after acid leaching, Cu content in it is 20.8 wt % and Cu2S and CuS are major copper compounds. Possible reactions were proposed for sulfuric acid baking of copper precipitate. HCN formed during baking is reoxidized to CO2 and N2, so recovery of cyanide during baking is of no significance. Based on this investigation, new process for treatment of copper precipitate is proposed for copper recovery. The process mainly consists of acid baking, acid leaching and SX-EW. And treatment of off-gas is needed in view of environmental issue.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


