动态环二硒引导手性前药自组装的锁-键生物识别驱动的强大抗肿瘤治疗
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
设计高选择性的纳米药物,精确识别生物界面,以实现有效的癌症治疗是一项巨大的挑战。受生物固有的手性和对映选择性的启发,我们构建了动态手性环二烯共轭紫杉醇前药纳米组装体(csepn)来模拟手性识别过程。通过解构csepn的锁-键生物识别机制,筛选具有有效抗肿瘤作用的最佳手性构型。与R-(−)- csep相比,S-(+)- csep表现出稳定的手性依赖自组装,这是由于分子间相互作用和空间位阻的平衡。S-(+)- csepn具有环张力骨架和优越的手性拓扑结构,具有超高的氧化还原敏感性和增强的网格蛋白介导的内吞作用。更重要的是,S-(+)- csepn具有肿瘤蓄积高、排泄率低的体内转运优势。最后,csepn通过化疗、肿瘤氧化还原轴调节和肿瘤血管生成抑制发挥了强大的协同肿瘤抑制作用。这些发现证实了手性锁键生物识别在决定纳米药物的生物学命运方面的主导作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
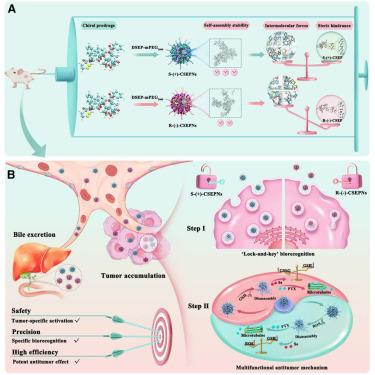
Robust antitumor treatment driven by lock-and-key biorecognition of dynamic cyclic diselenide-guided chiral prodrug self-assembly
Designing highly selective nanomedicines with precise recognition of biological interfaces for efficient cancer therapy represents a tremendous challenge. Inspired by the inherent chirality and enantioselectivity of organisms, we constructed dynamic chiral cyclic diselenide-conjugated paclitaxel prodrug nanoassemblies (CSEPNs) to simulate the chiral recognition process. The optimal chiral configuration with potent antitumor effects was screened by deconstructing the lock-and-key biorecognition of CSEPNs. Compared with R-(−)-CSEP, S-(+)-CSEP displayed steady chirality-dependent self-assembly due to the balance of intermolecular interaction and steric hindrance. With ring-tensioned backbone and superior chiral topology, S-(+)-CSEPNs exhibited ultra-high redox sensitivity and enhanced clathrin-mediated endocytosis. More importantly, S-(+)-CSEPNs presented the in vivo transport advantages of high tumor accumulation and low excretion rate. Finally, CSEPNs exerted robust synergistic tumor suppression through chemotherapy, tumor redox axis modulation, and tumor angiogenesis inhibition. These findings confirmed the dominant role of chiral lock-and-key biorecognition in determining the biological fate of the nanomedicines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


