紧凑的聚乙烯亚胺复合物mRNA疫苗
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
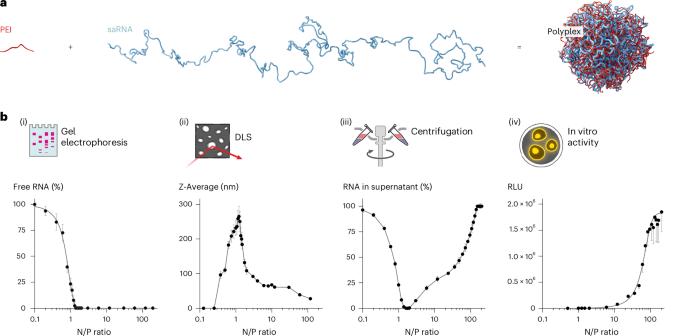
在这里,我们描述了配方包括个体,聚合物复杂的自我扩增RNA (saRNA)分子,设计用于预防传染病和其他预防和治疗应用的疫苗接种。当暴露于大量的阳离子聚合物聚乙烯亚胺(PEI)中时,溶液中的单个saRNA分子从扩展到球形组织进行重组,其特征是高堆积密度,低聚合物质量分数,因此,复合纳米颗粒的尺寸非常小,约为30纳米。与先前描述的saRNA/PEI配方相比,这种形式的PEI复合saRNA在体外和体内均表现出增强的生物活性。在疫苗接种模型中,可以在较低剂量下实现相关的免疫反应,为实际应用提供了潜在的优势。我们发现单个pei复合物RNA分子在一定程度上也存在于传统配方中。单分子部分与活性之间的直接关系表明,在不同的配方类型中,这种形式主要有助于活性。络合是由带相反电荷的聚电解质之间的自组装机制驱动的,这使得该协议广泛适用于各种阳离子聚合物和RNA结构。由于它们的小尺寸和在生物流体中的良好稳定性,这些紧凑的RNA分子也有望将遗传物质系统地传递到大颗粒难以到达的隔间。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Compact polyethylenimine-complexed mRNA vaccines
Here we describe formulations comprising individual, polymer-complexed self-amplifying RNA (saRNA) molecules, designed for vaccination against infectious diseases and other prophylactic and therapeutic applications. When exposed to a large excess of the cationic polymer polyethylenimine (PEI), the single saRNA molecules in solution reorganize from an extended to a globular organization, characterized by a high packing density, low polymer mass fraction and, consequently, a very small size of the polyplex nanoparticles of about 30 nm. This format of PEI-complexed saRNA exhibits enhanced biological activity in comparison with previously described saRNA/PEI formulations, both in vitro and in vivo. In vaccination models, relevant immune responses at lower doses are achieved, offering potential advantages for practical use. We found that the single PEI-complexed RNA molecules are also present in conventional formulations to some degree. The direct correlation between the single-molecule fraction with activity suggests that it is this format that predominantly contributes to activity in the different formulation types. Complexation is driven by mechanisms of self-assembly between oppositely charged polyelectrolytes, making this protocol broadly applicable to various cationic polymers and RNA constructs. With their small size and good stability in biofluids, these compacted RNA molecules are also promising for the systemic delivery of genetic material to compartments that are difficult to reach with larger particles. Single, self-amplifying RNA molecules condensed by an oppositely charged polyelectrolyte self-assemble into compact globular nanoparticles that can be used as vaccines to generate potent immunological responses at low doses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


