转录因子结合位点的遗传变异在很大程度上解释了玉米的表型遗传力
IF 29
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
转录因子(TF)结合位点(顺式元件)功能变异的综合图谱对于阐明基因型如何塑造表型至关重要。在这里,我们报道了在水分充足和干旱条件下玉米叶片泛雨的构建。我们在25个玉米杂交品种的泛基因组中量化了单倍型特异性TF足迹,并绘制了超过20万个与顺式元件占用相关的变异,遗传的,表观遗传的,或两者兼有(称为结合定量性状位点(bQTL))。三方面的证据支持bQTL的功能意义:(1)在干旱条件下,bQTL与vgt1、ZmTRE1和zmac111附近的MITE转座子等性状的致病位点重合;(2)近亲亲本间存在bQTL等位基因偏倚,且与染色质免疫沉淀测序结果相匹配;(3)跨基因组区域遗传变异的划分表明,bQTL捕获了143种表型中约72%的大部分可遗传性状变异。我们的研究为复杂性状的遗传研究和定向工程提供了一种可行的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
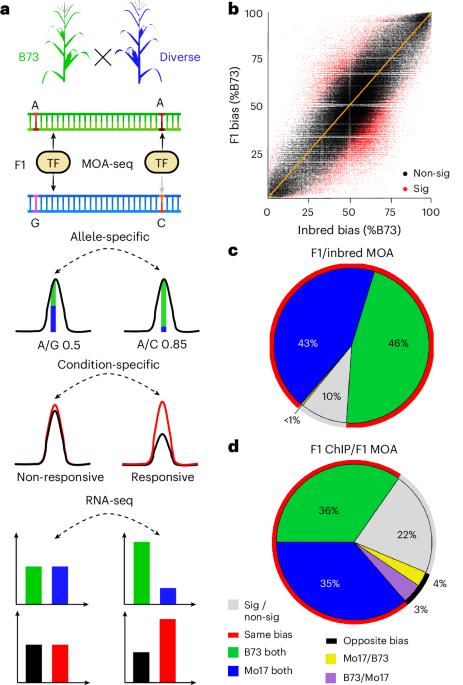
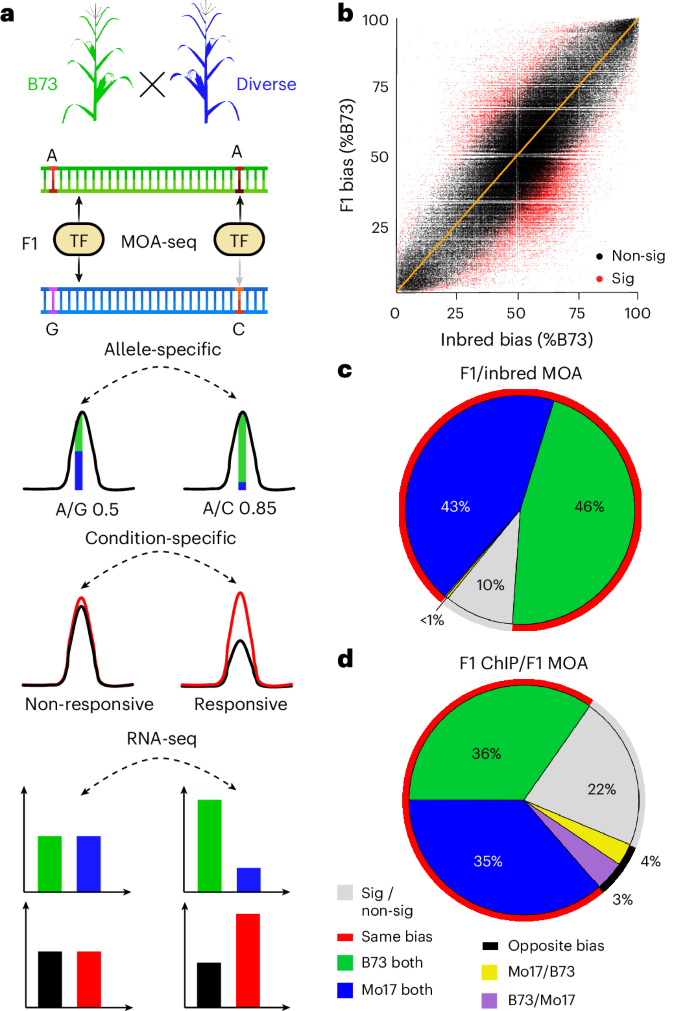
Genetic variation at transcription factor binding sites largely explains phenotypic heritability in maize
Comprehensive maps of functional variation at transcription factor (TF) binding sites (cis-elements) are crucial for elucidating how genotype shapes phenotype. Here, we report the construction of a pan-cistrome of the maize leaf under well-watered and drought conditions. We quantified haplotype-specific TF footprints across a pan-genome of 25 maize hybrids and mapped over 200,000 variants, genetic, epigenetic, or both (termed binding quantitative trait loci (bQTL)), linked to cis-element occupancy. Three lines of evidence support the functional significance of bQTL: (1) coincidence with causative loci that regulate traits, including vgt1, ZmTRE1 and the MITE transposon near ZmNAC111 under drought; (2) bQTL allelic bias is shared between inbred parents and matches chromatin immunoprecipitation sequencing results; and (3) partitioning genetic variation across genomic regions demonstrates that bQTL capture the majority of heritable trait variation across ~72% of 143 phenotypes. Our study provides an auspicious approach to make functional cis-variation accessible at scale for genetic studies and targeted engineering of complex traits. Pan-cistrome of the maize leaf under well-watered and drought conditions profiled by haplotype-specific MOA-seq highlights the relevance of transcription factor binding QTLs for understanding phenotypic diversity in maize.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


