成人海马神经发生的跨物种分析揭示了人类特异性基因表达,但趋同的生物学过程
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
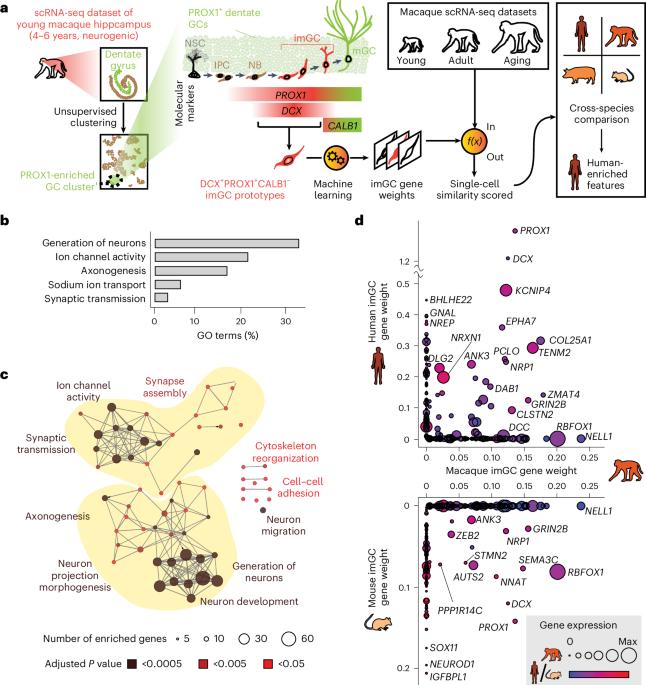
来自成年海马神经发生的未成熟齿状颗粒细胞(imGCs)有助于可塑性、学习和记忆,但它们在物种间的进化变化和人类的特化特征仍然知之甚少。在这里,我们对已发表的单核rna测序数据集进行了机器学习增强分析,并鉴定了具有转录组全未成熟神经元特征的猕猴图像gcs。我们在人类、猴子、猪和小鼠之间的跨物种比较显示,在imGCs中几乎没有共享的基因表达(如DPYSL5),但大多数是物种特异性基因表达,这些基因表达融合到调节神经元发育的共同生物过程中。我们进一步确定了人类特异性imgc的转录组学特征,并证明了富含人类imgc的质子运输液泡型atp酶亚型家族在人类多能干细胞衍生的imgc发育中的功能作用。我们的研究揭示了不同物种imGCs分子特征的不同基因表达模式和趋同的生物学过程,突出了对不同物种成人神经发生进行独立分子和功能分析的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cross-species analysis of adult hippocampal neurogenesis reveals human-specific gene expression but convergent biological processes
Immature dentate granule cells (imGCs) arising from adult hippocampal neurogenesis contribute to plasticity, learning and memory, but their evolutionary changes across species and specialized features in humans remain poorly understood. Here we performed machine-learning-augmented analysis of published single-nucleus RNA-sequencing datasets and identified macaque imGCs with transcriptome-wide immature neuronal characteristics. Our cross-species comparisons among humans, monkeys, pigs and mice showed few shared (such as DPYSL5), but mostly species-specific gene expression in imGCs that converged onto common biological processes regulating neuronal development. We further identified human-specific transcriptomic features of imGCs and demonstrated the functional roles of human imGC-enriched expression of a family of proton-transporting vacuolar-type ATPase subtypes in the development of imGCs derived from human pluripotent stem cells. Our study reveals divergent gene expression patterns but convergent biological processes in the molecular characteristics of imGCs across species, highlighting the importance of conducting independent molecular and functional analyses for adult neurogenesis in different species. Machine-learning-augmented single-nucleus transcriptomic analysis compared molecular landscapes of immature neurons in the mammalian hippocampus across species, highlighting human-specific gene expression but convergent biological processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


