胰腺-海马反馈机制调节抑郁相关行为的昼夜节律变化
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
神经精神障碍患者常表现出代谢症状。然而,这种共同发生的机制尚不清楚。本研究表明,来自双相情感障碍患者的诱导多能干细胞衍生的胰岛由于RORβ(一种双相情感障碍的易感基因)的表达增加而导致胰岛素分泌不足。增强小鼠胰腺β细胞中RORβ的表达可诱导抑郁相关行为在光明期和躁狂样行为在黑暗期。光照期胰腺RORβ过表达减少胰岛胰岛素释放,诱导海马过度活跃和抑郁样行为。此外,这种海马在光明期的过度活跃对促进胰岛素在黑暗期的释放有延迟作用,导致躁狂样行为和海马神经元的低活性。我们在小鼠身上的研究结果表明,代谢和昼夜节律因素合作产生行为波动的胰腺-海马体反馈机制可能在双相情感障碍中发挥作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

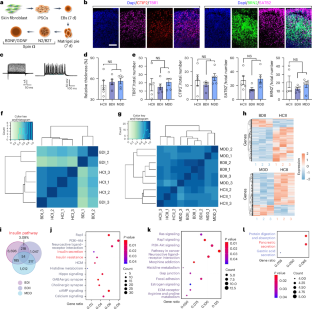
A pancreas–hippocampus feedback mechanism regulates circadian changes in depression-related behaviors
Individuals with neuropsychiatric disorders often show metabolic symptoms. However, the mechanisms underlying this co-occurrence remain unclear. Here we show that induced pluripotent stem cell-derived pancreatic islets from individuals with bipolar disorder have insulin secretion deficits caused by increased expression of RORβ, a susceptibility gene for bipolar disorder. Enhancing RORβ expression in mouse pancreatic β cells induced depression-related behaviors in the light phase and mania-like behaviors in the dark phase. Pancreatic RORβ overexpression in the light phase reduced insulin release from islets, inducing hippocampal hyperactivity and depression-like behaviors. Furthermore, this hippocampal hyperactivity in the light phase had the delayed effect of promoting insulin release in the dark phase, resulting in mania-like behaviors and hippocampal neuronal hypoactivity. Our results in mice point to a pancreas–hippocampus feedback mechanism by which metabolic and circadian factors cooperate to generate behavioral fluctuations and which may play a role in bipolar disorder. The mechanisms linking neuropsychiatric and metabolic disorders remain unclear. The authors show a pancreas–hippocampus feedback loop whereby metabolic and circadian factors drive behavioral fluctuations, with potential relevance for bipolar disorder.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


