芳基硫铵盐的电化学开环氧化研究
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
芳基硫铵盐的转化已成为获得精细化学品的通用方法。然而,这些方法主要集中在通过脱硫蒽法合成取代芳烃,这不可避免地导致了硫蒽的浪费。在这项工作中,在不需要化学氧化试剂的情况下完成了芳基硫铵盐的电化学开环氧化。该电化学方案不仅为制备芳基硫代二芳基亚砜提供了一条可靠的途径,也为进一步探索芳基硫代盐的合成用途提供了新的前景。更重要的是,所得到的亚砜产品可以用于生产不对称双硫化物、双亚砜和双砜,收率很高。机理研究和DFT计算表明,亚砜基团中的氧原子来自于水,氧自由基可能是芳基硫鎓盐开环过程中的关键中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
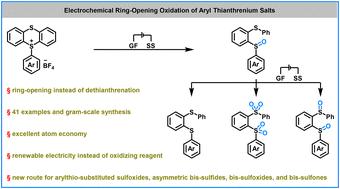
Electrochemical ring-opening oxidation of aryl thianthrenium salts†
Transformations of aryl thianthrenium salts have emerged as versatile approaches to access fine chemicals. However, these methods have mainly focused on the synthesis of substituted arenes via a dethianthrenation process, which inevitably leads to thianthrene waste. In this work, an interesting electrochemical ring-opening oxidation of aryl thianthrenium salts is accomplished without the need for chemical oxidizing reagents. This electrochemical protocol not only provides a robust route for obtaining arylthio-substituted diarylsulfoxides, but also offers new prospects for further exploring the synthetic utility of aryl thianthrenium salts. More importantly, the resulting sulfoxide products could be applied to produce asymmetric bis-sulfides, bis-sulfoxides, and bis-sulfones with excellent yields. Mechanistic studies and DFT calculations suggest that the oxygen atom in the sulfoxide group comes from H2O and the oxygen radical might be a key intermediate in the ring-opening process of aryl thianthrenium salts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


