单电子转移/氢原子转移协同催化绿色合成苄基硫酯†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
苄基硫酯是硫酯的一个特殊亚类,作为重要的中间体广泛应用于有机合成和医药领域。传统的苄基硫酯合成方法往往受到有毒硫源、条件恶劣、程序复杂或收率低的影响,存在固有的安全性和可持续性挑战。在此,我们描述了一种新型的协同光催化系统,该系统集成了基于金属硫族化合物簇的催化剂的单电子转移(SET)和基于多金属氧酸盐的催化剂的氢原子转移(HAT),实现了从现成的原料:单质硫、苄基氯化物和醛中绿色合成苄基硫酯。该方案通过可选的双硫自由基偶联途径进行操作,并显示出非常广泛的底物范围,即与各种苄基氯化物和醛兼容,从而实现原子合成、步进合成和氧化还原经济合成。此外,该系统还展示了多种复杂天然产物的后期功能化和目标生物活性分子的合成,如潜在的硫化氢供体和蛇毒拮抗剂。我们的工作为合成苄基硫酯提供了一种绿色光催化方法,为有机和药物合成中多种C-S键的构建开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
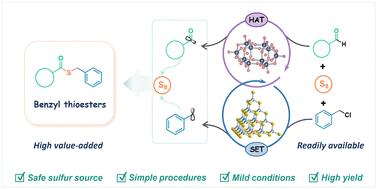
Single electron transfer/hydrogen atom transfer synergistic catalysis for green synthesis of benzyl thioesters†
Benzyl thioesters, a specific subclass of thioesters, are widely used as important intermediates in organic synthesis and the pharmaceutical field. Traditional synthetic methods for benzyl thioesters often suffer from toxic sulfur sources, harsh conditions, complex procedures, or low yields, posing inherent safety and sustainability challenges. Herein, we describe a novel synergistic photocatalytic system that integrates single electron transfer (SET) of a metal-chalcogenide-cluster-based catalyst and hydrogen atom transfer (HAT) of a polyoxometalate-based catalyst, achieving the green synthesis of benzyl thioesters from readily available feedstocks: elemental sulfur, benzyl chlorides, and aldehydes. This protocol operates via an optional dual sulfur radical-based coupling pathway and exhibits a remarkably broad substrate scope, i.e. compatible with a wide variety of benzyl chlorides and aldehydes, enabling atom-, step-, and redox-economical synthesis. Furthermore, the system demonstrates the late-stage functionalization of various complex natural products and the synthesis of target bioactive molecules, such as a potential hydrogen sulfide donor and a snake venom antagonist. Our work offers a green photocatalytic method for the synthesis of benzyl thioesters and opens new avenues for the construction of diverse C–S bonds in organic and pharmaceutical syntheses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


