低能耗和绿色原位回收废旧锂离子电池,实现石墨再生和预锂化†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
锂离子电池中不可再生石墨负极材料的循环再生是电池回收利用的关键,旨在减少碳足迹,最大限度地减少资源浪费。传统的方法往往涉及高能耗和严重的环境污染。为了解决这个问题,开发了一种仅使用草酸的绿色闭环再生方法。这种方法有效地从阳极中回收贵金属,并允许废阳极的原位再生,以及预锂化试剂的合成。研究结果表明,预锂化再生石墨表面的草酸锂补偿了活性Li+的损失,在电池运行过程中起到了预锂化牺牲盐的作用。它还分解释放二氧化碳,防止可溶性锂烷氧化物的形成和电解质酯交换反应,显著提高电池性能而不留下杂质。这种方法允许从废阳极和原位闭环再生中有效利用锂。在最优条件下,Li+浸出率可达97.64%。经过200次循环后,再生预锂化石墨的电化学性能保持在320.5 mAh g−1的高比容量,比容量保持率为90.4%。环境和经济评估显示,回收每吨退役石墨的突出经济利润为3259.22美元,环境足迹可以忽略不计。这项工作促进了材料回收,减少了废电池对环境的影响,并支持了低碳转型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
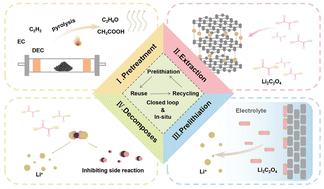
Low-energy and green in situ recycling of spent lithium-ion batteries to achieve graphite regeneration and pre-lithiation†
The cyclic regeneration of non-renewable graphite anode materials in lithium-ion batteries (LIBs) is crucial for battery recycling, aiming to reduce carbon footprints and minimize resource waste. Traditional methods often involve high energy use and significant environmental pollution. To tackle this, a green, closed-loop regeneration method using only oxalic acid was developed. This approach efficiently recovers precious metals from the anode and allows for in situ regeneration of the spent anode, as well as the synthesis of pre-lithiation reagents. The findings indicate that lithium oxalate on the surface of pre-lithiated regenerated graphite compensates for active Li+ loss and acts as a pre-lithiation sacrificial salt during battery operation. It also decomposes to release carbon dioxide, preventing the formation of soluble lithium alkoxides and electrolyte ester exchange reactions, significantly enhancing battery performance without leaving impurities. This method allows effective lithium utilization from spent anodes and in situ closed-loop regeneration. Under optimal conditions, the Li+ leaching rate reaches 97.64%. The electrochemical performance of the regenerated prelithiated graphite maintains a high specific capacity of 320.5 mAh g−1 after 200 cycles, with a specific capacity retention rate of 90.4%. Environmental and economic assessment shows an outstanding economic profit of $3259.22 and a negligible environmental footprint for recycling each ton of retired graphite. This work advances material recycling, reduces the environmental impact of waste batteries, and supports a low-carbon transition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


