碳碳双键的有机电还原
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
有机电化学合成已成为一种实用、环保的合成方法,广泛应用于氧化、还原和氧化还原中性反应。通过精确控制电流或电势,可以在温和的反应条件下实现一些具有挑战性的化学转化。由于日益严峻的能源和环境问题,电化学合成成为近年来研究的热点。然而,由于有机电合成中还原反应的研究相对于氧化反应的研究滞后,本文主要对有机电合成中的阴极还原反应进行综述。我们从发生在碳碳双键上的两种类型的反应开始,还原性氢化(氘化)和还原性偶联,介绍有机合成和电化学的迷人交集。通过回顾最新进展,读者可以深入了解有机电合成中的阴极还原反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
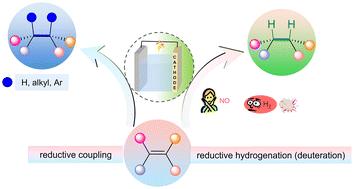
Organic-electrical reduction of carbon–carbon double bonds
Organic electrochemical synthesis has become a practical and environmentally friendly synthesis method and is widely used in oxidation, reduction, and redox-neutral reactions. By precisely controlling the current or potential, it is possible to achieve some challenging chemical transformations under mild reaction conditions. Owing to increasingly severe energy and environmental issues, electrochemical synthesis has become the focus of research in recent years. However, due to the lag in research on reduction reactions compared to oxidation reactions in organic electrosynthesis, the main focus of this review is on cathodic reduction reactions in organic electrosynthesis. We start with two types of reactions that occur on carbon–carbon double bonds, reductive hydrogenation (deuteration) and reductive coupling, to introduce the fascinating intersection of organic synthesis and electrochemistry. By reviewing the latest developments, readers can gain an in-depth understanding of cathodic reduction reactions in organic electrosynthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


