构建CuO-CeO2异质界面增强低温CO氧化和SO2抗性
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
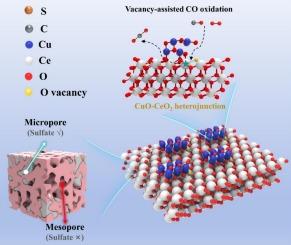
采用浸渍法制备了低温CO氧化用CuO-CeO2复合催化剂。制备的CuO-CeO2复合材料在约~ 70℃和~ 110℃(记为T50和T100)下分别达到50%和100%的转化率。催化活性的增强是由于形成了丰富的CuO/CeO2异质结构,增加了其晶格中的氧空位。此外,在浸渍合成过程中,还对CuO-CeO2复合材料的孔隙结构进行了优化。与仅具有微孔的裸CeO2相比,CuO-CeO2在其表面形成了更多的介孔,这有助于降低硫酸盐在表面的沉积速率。催化剂的SO2耐受性,因此其稳定性对实际工业烟气处理显著提高。此外,利用密度泛函理论(DFT)探讨了CO对CuO-CeO2的氧化机理。结果表明,CuO/CeO2异质界面上容易形成氧空位;CO的吸附能(ΔG)因此降低到0.15 eV,远低于裸CeO2 (0.53 eV)。引入CuO后,CeO2中Ce的f电子中心由- 0.55 eV上升到- 1.71 eV,表明Ce中的f电子变得更加活跃,有利于CO的吸附。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Constructing CuO-CeO2 heterointerfaces for enhanced Low-Temperature CO oxidation and SO2 resistance
CuO-CeO2 composite catalyst for low temperature CO oxidation was fabricated via an impregnation method. The prepared composite CuO-CeO2 achieved 50 % and 100 % conversion at approximately ∼ 70℃ and ∼ 110℃ (denoted as T50 and T100), respectively. The enhanced catalytic activities were attributed to the formation of abundant CuO/CeO2 heterostructures, which increased the concentration of oxygen vacancies in their lattices. In addition, the pore structure of the CuO-CeO2 composite has also been optimized during the impregnation synthesis process. Compared with bare CeO2, which only possesses micropores, CuO-CeO2 forms more mesopores on its surface, which assists in reducing the deposition rate of sulfate species on the surface. The SO2 tolerance of the catalyst and hence its stability towards practical industrial flue gas treatment is significantly promoted. Furthermore, Density Functional Theory (DFT) was also performed to explore the mechanism of CO oxidation on the CuO-CeO2. It was found that the oxygen vacancy can be easily formed on the CuO/CeO2 heterointerfaces; the adsorption energy (ΔG) of CO was therefore decreased to 0.15 eV, much lower than that of bare CeO2 (0.53 eV). Besides, the center of f electrons of Ce in CeO2 rises from −0.55 eV to – 1.71 eV by introducing CuO, suggesting that the f electrons in Ce become more active, which is beneficial for CO to adsorb.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


