脂肪酸白蛋白偶联物机械地削弱和破坏纤维蛋白网络的降解。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
心血管疾病和中风是西方国家最大的死亡原因。这些疾病与血栓的形成直接相关,血栓阻碍了重要器官的血液流动。这种血栓的常见危险因素是肥胖、高血压、糖尿病和高低密度脂蛋白胆固醇。立即溶栓治疗溶解富含纤维蛋白的凝块可以挽救生命,但最近的研究表明,纯脂肪酸(FA)可以通过与纤溶蛋白的相互作用抑制溶栓。然而,由于FAs经常与血液中的血清白蛋白络合,因此尚不清楚血液中的FAs如何与纤溶蛋白或凝块相互作用以调节溶栓。在这里,我们研究了两种丰富的FAs(油酸和棕榈酸)与牛血清白蛋白(BSA)复合物的水平升高如何影响纤维蛋白水凝胶及其降解。我们通过Förster共振能量转移显微镜观察了脂肪酸- bsa (FABSA)偶联物与纤维蛋白的结合,并注意到与纯纤维蛋白网络相比,FABSA对纤维蛋白凝胶力学和纤维蛋白溶解的影响。具体来说,由于纤维蛋白网络中存在FABSA,纤维蛋白水凝胶被机械削弱,纤维蛋白溶解速度显著降低。这些研究表明,脂肪酸含量的升高改变了凝块的性质,使它们在机械上更弱,更不易降解。意义声明:纤维蛋白网络是血凝块的主要承载元素,它在体内受到各种循环力的影响,并且由于纤维蛋白的层次结构而具有优异的机械性能。这些网络是在血液中脂肪酸、脂蛋白和白蛋白存在的情况下形成的;然而,人们对这些额外成分如何改变纤维蛋白网络特性知之甚少。通过对蛋白纤维取向、分子结构和原位降解的成像,我们发现在与白蛋白结合的脂肪酸升高的情况下,纤维蛋白的形成改变了网络结构,机械地削弱了纤维蛋白网络,并大大减缓了纤维蛋白网络的降解。根据我们的发现,我们怀疑血液中脂肪的增加可能导致纤维蛋白降解受阻和血栓持久性增加。本文章由计算机程序翻译,如有差异,请以英文原文为准。
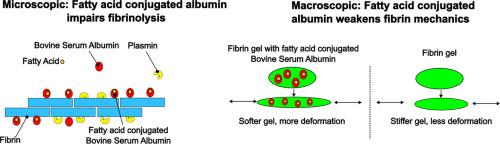
Fatty acid albumin conjugates mechanically weaken and disrupt degradation of fibrin networks
Cardiovascular diseases and stroke together account for the largest causes of death in Western countries. These pathologies are directly linked to the formation of blood clots that block blood flow to vital organs. Common risk factors for such clots are obesity, high blood pressure, diabetes, and high LDL cholesterol. Immediate thrombolytic treatment to dissolve fibrin-rich clots can save lives, but recent research has shown that pure fatty acids (FA) can inhibit thrombolysis via interaction with plasmin. However, since FAs are often complexed to serum albumin in the blood, it is unclear how FAs in blood interact with plasmin, or perhaps with clots, to modulate thrombolysis. Here, we studied how elevated levels of two abundant FAs (oleic and palmitic acid) complexed to bovine serum albumin (BSA) affect fibrin hydrogels and their degradation. We observed the binding of fatty acid-BSA (FABSA) conjugates to fibrin via Förster resonance energy transfer microscopy and noted the effects of FABSA on fibrin gels mechanics and fibrinolysis compared to pure fibrin networks. Specifically, with FABSA in the fibrin network, fibrin hydrogels were mechanically weakened and showed a significant decrease in fibrinolysis speed. These studies show that elevated fatty acid content modifies clot properties, making them mechanically weaker and more resistant to degradation.
Statement of significance
Fibrin networks are the primary load-bearing element in blood clots, which are subject to various cyclic forces in the body and have excellent mechanical properties resulting from fibrin’s hierarchical structure. These networks are formed in the presence fatty acids, lipoproteins, and albumin in the blood; however, little is known about how these additional constituents modify fibrin network properties. By imaging protein fiber orientation, molecular structure, and degradation in situ, we find that fibrin formation in the presence of elevated fatty acids conjugated to albumin modifies network structure, mechanically weakens fibrin networks, and slows fibrin network degradation considerably. Based on our findings, we suspect that increased fat in blood may lead impeded fibrin degradation and increased clot persistence.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


