HFPO-TA通过破坏铁代谢稳态诱导斑马鱼肝损伤和铁下垂。
IF 4.3
3区 环境科学与生态学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology C-toxicology & Pharmacology
Pub Date : 2025-08-06
DOI:10.1016/j.cbpc.2025.110318
引用次数: 0
摘要
六氟环氧丙烷三聚酸(HFPO-TA)是全氟辛酸(PFOA)的一种新型替代品,其毒性与全氟辛酸相似。然而,HFPO-TA是否导致铁下垂目前尚不清楚。本实验系统研究了HFPO-TA诱导斑马鱼胚胎肝脏铁下垂对肝损伤的影响。然后通过苏丹黑B和H&E染色观察肝脏形态和炎症损伤程度,并观察Tg (fabp10a: DsRed), Tg (lyz: DsRed)和Tg (Xla)。Eef1a1: mlsEGFP)转基因斑马鱼。利用先前在实验室完成的转录组学数据,鉴定了与铁下垂相关的差异表达基因,并从中选择了三个受体作为与HFPO-TA分子对接的受体,受体和配体都能够成功对接。然后我们在显微镜下分别用ROS、DPPP和FerroOrange染色观察斑马鱼。通过RT-qPCR验证关键基因的表达。结果表明,HFPO-TA可导致ROS、脂质过氧化物和二价铁离子水平显著升高,肝脏炎症和损伤,tfa、tfr1a等差异基因有升高趋势。然后,我们使用激动剂(RSL3)和抑制剂(Fer-1)验证了铁下垂途径,发现抑制剂能够改善hfpo - ta诱导的炎症和铁下垂反应,而激动剂则相反。总之,HFPO-TA诱导铁下垂导致肝损伤的能力为HFPO-TA毒性作用的机制提供了新的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
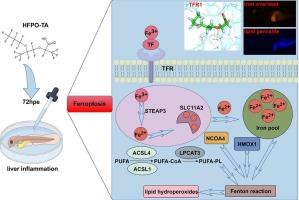
HFPO-TA induces liver damage and ferroptosis in zebrafish by disrupting iron metabolism homeostasis
Hexafluoropropylene oxide trimeric acid (HFPO-TA) is a novel alternative to perfluorooctanoic acid (PFOA) with similar toxicity to PFOA. However, whether HFPO-TA causes ferroptosis is currently unknown. In this experiment, we systematically investigated the induction of zebrafish embryonic liver ferroptosis by HFPO-TA to cause liver injury. The liver morphology and the extent of inflammatory damage were then visualized by Sudan Black B and H&E staining, as well as through observations of Tg (fabp10a: DsRed), Tg (lyz: DsRed) and Tg (Xla.Eef1a1: mlsEGFP) transgenic zebrafish. Differentially expressed genes associated with ferroptosis were identified using transcriptomics data previously done in the lab, and from these, three were selected as receptors for molecular docking with HFPO-TA, and both receptors and ligands were able to dock successfully. We then observed zebrafish under the microscope using ROS, DPPP and FerroOrange staining, respectively. The expression of key genes was verified by RT-qPCR. The results showed that HFPO-TA could lead to a significant increase in the levels of ROS, lipid peroxides and divalent iron ions, liver inflammation and injury, and a trend of elevated differential genes such as tfa and tfr1a. We then validated the ferroptosis pathway using an agonist (RSL3) and an inhibitor (Fer-1), and found that the inhibitor was able to ameliorate HFPO-TA-induced inflammatory and ferroptosis responses, whereas the agonist did the opposite. In conclusion, the ability of HFPO-TA to induce ferroptosis, which leads to liver injury provides new insights into the mechanism of HFPO-TA toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.50
自引率
5.10%
发文量
206
审稿时长
30 days
期刊介绍:
Part C: Toxicology and Pharmacology. This journal is concerned with chemical and drug action at different levels of organization, biotransformation of xenobiotics, mechanisms of toxicity, including reactive oxygen species and carcinogenesis, endocrine disruptors, natural products chemistry, and signal transduction with a molecular approach to these fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


