黑腹果蝇含Dα2烟碱受体正位结合位点突变的功能影响表明,Dβ1环D中的精氨酸比Dα2环C中的引入丝氨酸对新烟碱选择性的贡献更大。
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
迄今为止的研究表明,某些正位结合位点参与了新烟碱类对昆虫烟碱乙酰胆碱受体(nAChRs)的选择性作用。然而,很少有研究利用仅由昆虫nAChR亚基组成的功能性nAChR。对于选择性新烟碱作用的机制,环C已被证明发挥了作用,在此,我们研究了Dα2亚基C环P242E和P242S突变和Dβ1亚基D环R81T突变对吡虫啉和噻虫啉对黑腹果蝇Dα1/Dα2/Dβ1和Dα1/Dα2/Dβ1/Dβ2 nAChRs与辅助因子DmNACHO、DmRIC-3、DmTMX3和DmUNC-50共表达的nAChRs在非洲爪蟾卵细胞中的受体活性的影响。环C的P242E和P242S突变几乎不影响新烟碱类激动剂的亲和力和药效。相反,环D中的R81T突变降低了吡虫啉的亲和力和药效,同时大幅降低了噻虫啉的药效。环C和环D的联合突变导致噻虫啉的药效进一步降低,而对吡虫啉的作用没有影响。这些结果表明,C环上的脯氨酸和D环上的精氨酸是新烟碱结合的基础,其中D环的贡献更大,与吡虫啉相比,噻虫啉对D环上精氨酸的相互作用依赖更小,因此在抗性发展过程中对R81T突变的敏感性更低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
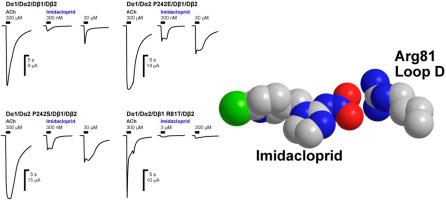
The functional impact of mutations in orthosteric binding site of the Drosophila melanogaster Dα2-containing nicotinic receptors points to a greater contribution to neonicotinoid selectivity of an arginine in loop D of Dβ1 than an introduced serine in loop C of Dα2
Studies to date show that certain orthosteric binding sites are involved in the selective actions of neonicotinoids on insect nicotinic acetylcholine receptors (nAChRs). However, few investigations have utilized functional nAChRs consisting only of insect nAChR subunits. For the mechanism of selective neonicotinoid actions, loop C has been shown to play a role, whereas functional studies have advocated roles for loop D and loop G which appear to contribute more strongly to neonicotinoid actions than loop C. Here we have investigated the effects of P242E and P242S mutations in loop C of the Dα2 subunit and R81T mutation in loop D of the Dβ1 subunit on agonist actions of imidacloprid and thiacloprid for Drosophila melanogaster Dα1/Dα2/Dβ1 and Dα1/Dα2/Dβ1/Dβ2 nAChRs coexpressed with cofactors DmNACHO, DmRIC-3, DmTMX3 and DmUNC-50 in Xenopus laevis oocytes. The P242E and P242S mutations in loop C hardly affected the agonist affinity and efficacy of the neonicotinoids. In contrast, the R81T mutation in loop D decreased the affinity and efficacy of imidacloprid while substantially reducing the efficacy of thiacloprid. Combined loop C and loop D mutations resulted in further reduced efficacy of thiacloprid while having no such effect on imidacloprid actions. These results suggest that the proline in loop C and the arginine in loop D underly the binding of neonicotinoids with the greater contribution coming from loop D, and that thiacloprid relies less on the interactions with the arginine in loop D than imidacloprid, and hence is less susceptible to the R81T mutation in the development of resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


