甾醇改性植物通过限制甾醇的可用性来降低蚜虫的表现。
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
甾醇是真核细胞结构和代谢所必需的,但昆虫不能从头合成它们,必须通过饮食获得它们。对于食草昆虫来说,植物衍生的甾醇通常会转化为胆固醇来支持发育和繁殖。我们之前用沉默的HYD1基因改造拟南芥,导致甾醇成分发生改变。在本研究中,我们评估了韧皮部取食蚜虫对这些甾醇改性植物的作用。在改良系(HYD1RNAi10、12、25)上饲养的蚜虫的生长、繁殖和存活率明显低于野生型Col-0。然而,选择分析和电渗透(EPG)显示,寄主偏好或探测和摄食行为没有差异。与咀嚼昆虫小菜蛾不同,桃蚜不积累非典型甾醇,但总甾醇含量显著降低。韧皮部汁液分析反映了蚜虫的固醇谱,缺乏在改良系叶片组织中发现的非典型固醇。HYD1RNAi系的RNA-seq显示未诱导已知的植物防御途径;相反,参与翻译和硝酸盐代谢的基因被上调。这些发现表明,HYD1沉默通过限制昆虫发育和繁殖所需的甾醇来降低寄主对蚜虫的适应性。我们的研究结果强调了甾醇修饰植物作为一种有前途的策略来管理韧皮部取食汁液的害虫的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
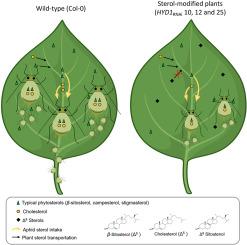
Sterol-modified plants reduce aphid performance by limiting sterol availability
Sterols are essential for eukaryotic cell structure and metabolism, yet insects cannot synthesize them de novo and must acquire them through their diet. For insect herbivores, plant-derived sterols are typically converted into cholesterol to support development and reproduction. We previously engineered Arabidopsis thaliana lines with silenced HYD1, resulting in altered sterol composition. In this study, we evaluated the performance of the phloem-feeding aphid Myzus persicae on these sterol-modified plants. Aphids reared on the modified lines (HYD1RNAi10, 12, 25) exhibited significantly reduced growth, reproduction, and survival compared to those on wild-type Col-0. However, choice assays and electropenetrography (EPG) revealed no differences in host preference or probing and feeding behaviors. Unlike the chewing insect Plutella xylostella, M. persicae did not accumulate atypical sterols but instead showed a significant reduction of total sterol content. Phloem-sap analysis mirrored aphid sterol profiles, lacking the atypical sterols found in leaf tissue of the modified lines. RNA-seq of HYD1RNAi lines revealed no induction of known plant defense pathways; instead, genes involved in translation and nitrate metabolism were upregulated. These findings show that HYD1 silencing reduces host suitability for aphids by limiting sterol availability for insect development and reproduction. Our results highlight the potential of sterol-modified plants as a promising strategy for managing phloem sap-feeding insect pests.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


