镍催化甲基环丙烷向氰基取代的季碳中心开环氢化反应
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
过渡金属催化的亚甲基环丙烷的氢功能化反应是一种有用的转化,通常产生1,2-氢功能化产物作为主要产物。在这项工作中,我们报道了前所未有的,高选择性镍催化的亚甲基环丙烷氢氰化反应,该反应提供1,4-氢化产物,形成无环氰基取代的季碳中心。机理研究表明,该反应通过涉及二烯中间体的β-碳消除过程进行。DFT计算进一步表明,在C-CN还原消除过程中,四元碳中心形成的区域选择性受到空间和非共价相互作用的微妙相互作用的控制本文章由计算机程序翻译,如有差异,请以英文原文为准。
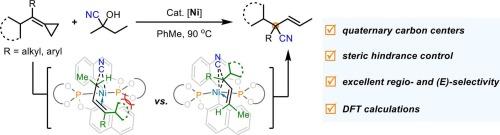
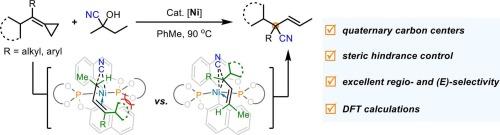
Ni-catalyzed ring-opening hydrocyanation of methylenecyclopropanes toward cyano-substituted quaternary carbon centers
Transition-metal-catalyzed hydrofunctionalization of methylenecyclopropanes represents a useful transformation, typically yielding 1,2-hydrofunctionalization products as the major outcome. In this work, we report an unprecedented, highly selective nickel-catalyzed hydrocyanation of methylenecyclopropanes that affords 1,4-hydrofunctionalization products, forming acyclic cyano-substituted quaternary carbon centers. Mechanistic studies suggest that this reaction proceeds via a β-carbon elimination process involving a diene intermediate. DFT calculations further revealed that the regioselectivity for quaternary carbon center formation was controlled by a delicate interplay of steric and noncovalent interactions during the C–CN reductive elimination step.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


