无定形碳酸锂作为锂在无机固体电解质界面中的优越输运途径
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
锂离子在电极-电解质界面上的空间均匀和便捷的传输是实现高能量密度锂离子电池循环耐久性的关键。在这项工作中,我们使用一系列从头计算研究了无机非晶固体电解质间相层内锂离子的输运性质和机制。虽然与无缺陷的晶体相比,无定形Li2O和Li2CO3表现出优异的锂输运特性,但我们发现,这些无定形相中的易锂输运源于锂周围无序的氧环境,创造了普遍的类似缺陷的条件,促进了锂的集体输运。特别是,我们证明了由于锂和氧之间的库仑耦合较弱,与无定形Li2O相比,无定形Li2CO3作为一种优越的锂输运材料。我们进一步证明,锂在无定形Li2CO3中的输运速率可以进一步提高,因为锂的富锂成分取决于电池的工作条件,并且锂周围的氧配位降低。除了证实无定形无机固体电解质界面材料促进锂的输运外,我们的理论研究表明,无定形Li2CO3具有异常高的锂输运性能。我们阐明了与电池操作环境相关的这种卓越性能的潜在科学原理。对于自然形成或人工生成的钝化层如何影响下一代锂离子电池的性能,这一改进的理解提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
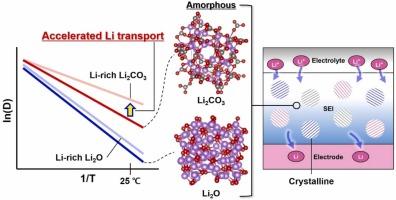
Amorphous lithium carbonate as a superior lithium transport pathway in inorganic solid electrolyte interphases
Spatially uniform and facile lithium transport at the electrode-electrolyte interface is key to achieving cycle durability in lithium-ion batteries with high energy density. In this work, we investigate the lithium-ion transport properties and mechanisms within inorganic amorphous solid electrolyte interphase layers using a series of ab initio calculations. While amorphous Li2O and Li2CO3 are found to exhibit excellent lithium transport properties compared to their defect-free crystalline counterparts, we find that the identified facile lithium transport in these amorphous phases stems from disordered oxygen environments around lithium, creating pervasive defect-like conditions that facilitate collective lithium transport. In particular, we demonstrate that amorphous Li2CO3 acts as a superior lithium transport material compared to amorphous Li2O due to weaker Coulomb couplings between lithium and oxygen. We further demonstrate that lithium transport rates in amorphous Li2CO3 can be further enhanced, as lithium-rich compositions occur depending on battery operating conditions and oxygen coordination around lithium is reduced. Beyond confirming that amorphous inorganic solid electrolyte interphase materials facilitate lithium transport, our theoretical study demonstrates that amorphous Li2CO3 exhibits exceptionally high lithium transport properties. We elucidate the underlying scientific principles responsible for this superior performance in correlation with battery operating environments. The improved understanding offers valuable insights into how naturally formed or artificially generated passivation layers affect the performance of next-generation lithium-ion batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


