脲基改性镁基吸附剂对氟化氢气体的吸附机理:氧空位的关键作用
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
氟化氢(HF)气体是有色金属冶炼、铝电解、煤炭燃烧和垃圾焚烧过程中的主要燃烧副产物。由于其高腐蚀性和毒性,对人类健康和环境生态系统构成严重威胁,因此有必要严格控制工业过程中的HF排放或采用适当的方法去除。本研究通过一锅法成功合成了含氧空位镁基吸附剂2NC@Mg(NO3) 2-500。该吸附剂的最大HF吸附容量为195.65 mg·g−1(效率为90% %),大大超过了先前文献报道的值。氧化镁(MgO)的碱性位点是促进HF解离的关键,而氧空位则促进HF吸附并转化为氟化镁(MgF2)。密度泛函理论(DFT)计算进一步证实了实验结果,表明氧空位通过稳定空位位置的氟离子(F-)显著增强了MgO对HF的吸附。随后这些空位的消耗和MgO向MgF2的转化导致吸附剂失活。该研究强调了氧空位在HF气体吸附中的重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
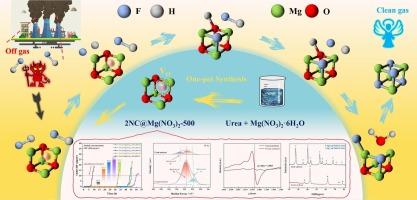
Adsorption mechanisms of hydrogen fluoride gas by urea-modified Mg-based adsorbent: The key role of oxygen vacancies
Hydrogen fluoride (HF) gas is a major combustion byproduct in non-ferrous metal smelting, aluminum electrolysis, coal combustion, and waste incineration processes. Due to its high corrosivity and toxicity, which pose a serious threat to human health and environmental ecosystems, it is necessary to strictly control HF emissions from industrial processes or remove it using proper methods. In this study, an oxygen vacancy-containing magnesium-based adsorbent (2NC@Mg(NO3)2–500) was successfully synthesized via a one-pot method. This adsorbent demonstrated a maximum HF adsorption capacity of 195.65 mg·g−1 (at 90 % efficiency), significantly exceeding values reported in previous literature. The basic sites of magnesium oxide (MgO) were critical for promoting HF dissociation, while oxygen vacancies facilitated HF adsorption and its conversion to magnesium fluoride (MgF2). Density functional theory (DFT) calculations further confirmed the experimental findings, demonstrating that oxygen vacancies significantly enhance HF adsorption on MgO by stabilizing fluoride ions (F-) at the vacancy sites. The subsequent consumption of these vacancies and the conversion of MgO to MgF2 led to adsorbent deactivation. This study highlights the important role of oxygen vacancies in HF gas adsorption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


