中性水电解将绿色氢气和可再生天然气从沼气中生产结合起来
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
将剩余的可再生电力转化为绿色氢(H2)和可再生天然气(RNG,即CH4)的电制气方法代表了有前途的储能技术,具有长期储气性、多功能性和低碳足迹的特点。提出了一种新的电制气方法,通过中性水电解(NWE)将沼气中的H2和RNG结合起来。该工艺利用水电解反应的固有能力,从ph中性电解质中产生酸和碱,促进ph介导的碳捕获和释放。NWE实现了与基准碱性水电解(AWE)电池相同的H2产率和纯度,同时将合成沼气(65 % CH4, 35 % CO2)升级为管道准备的可再生天然气,最大CH4浓度为99 %。NWE在二氧化碳去除方面的额外能耗(相对于AWE)达到了具有经济竞争力的水平,低至1.03 kWh/kgCO2(不包括泵的能源需求),同时得益于无化学物质的运行,没有废盐水的产生,紧凑的设计(与AWE相同),以及完全电解pH控制,而不需要昂贵的限流双极膜。本文章由计算机程序翻译,如有差异,请以英文原文为准。
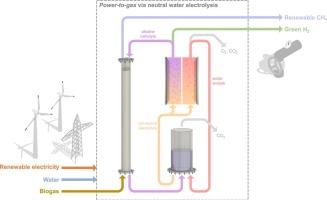
Neutral water electrolysis unifies green hydrogen and renewable natural gas production from biogas
Power-to-gas approaches that convert surplus renewable electricity into green hydrogen (H2) and renewable natural gas (RNG, i.e., CH4) represent promising energy storage technologies, featuring long-term gas storability, versatility, and low carbon footprint. A new power-to-gas approach is presented that couples H2 and RNG production from biogas via neutral water electrolysis (NWE). The process leverages the inherent capability of the water electrolysis reaction to produce acids and bases from pH-neutral electrolytes, facilitating pH-mediated carbon capture and release. NWE achieved the same H2 production rates and purities as a reference alkaline water electrolysis (AWE) cell, while concurrently upgrading synthetic biogas (65 % CH4, 35 % CO2) to pipeline-ready RNG with maximum CH4 concentrations of >99 %. NWE's added energy consumption for CO2 removal (relative to AWE) achieved economically competitive levels as low as 1.03 kWh/kgCO2 (excluding pump energy demand), while simultaneously benefitting from chemical-free operation with no waste brine production, a compact design (same as AWE), and exclusively electrolytic pH control without the need for costly and current-limiting bipolar membranes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


