超声激活压电水凝胶支架在加速伤口愈合中的协同免疫调节和血管生成。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
目前的伤口治疗努力动态调节免疫反应和血管生成,经常导致愈合受损,瘢痕形成和组织再生不良。智能水凝胶支架的发展为精确调节伤口愈合过程提供了机会。在这里,我们提出了一种开创性的无线免疫调节策略,通过将氨基修饰钛酸钡(BTN)纳米颗粒与天然胶原基质结合,使用氧化结冷胶(OG)作为交联剂,制造罗非鱼胶原(Col)基仿生压电水凝胶支架(Col/OG/BTN)。水凝胶支架具有类似皮肤的机械性能、可控的生物降解性和超声(US)激活的压电性,同时为细胞迁移和信号传递提供了三维多孔微环境。转录组学显示,在US下,水凝胶支架通过调节磷酸肌肽3激酶(PI3K) /蛋白激酶B (Akt)和肿瘤坏死因子(TNF)信号通路,将促炎M1巨噬细胞重编程为促愈合M2巨噬细胞。这种免疫调节与内皮细胞串扰协同作用,增强促血管生成因子的分泌。重要的是,在体内应用Col/OG/BTN水凝胶支架可显著减少炎症,促进血管生成,促进胶原沉积,刺激毛囊再生,最终实现高质量的伤口愈合和功能恢复。总之,本研究展示了一种时空可控的方法来调节炎症伤口的免疫微环境,同时促进血管再生,为再生医学提供了一种临床可翻译的策略。意义声明:目前的伤口治疗在动态调节免疫反应和血管生成方面面临挑战。我们开发了一种罗非鱼胶原基压电水凝胶支架,结合氧化结冷胶和氨基修饰钛酸钡纳米颗粒(Col/OG/BTN水凝胶支架)。这种超声激活的系统通过PI3K/Akt和TNF途径将促炎巨噬细胞重新编程为促愈合表型,协同促进血管生成和毛囊再生。该支架消除了植入电极,提供无线免疫调节和血管修复,使高质量的伤口愈合与功能性皮肤附着物恢复。这项工作为通过生物电信号修复炎症伤口提供了一种临床可翻译的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
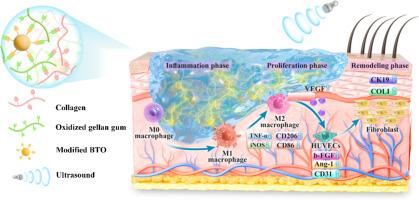
Ultrasound-activated piezoelectric hydrogel scaffold for synergistic immunomodulation and angiogenesis in accelerated wound healing
Current wound therapies struggle to dynamically regulate immune responses and angiogenesis, often resulting in impaired healing, scarring, and poor tissue regeneration. The development of smart hydrogel scaffolds offers an opportunity to precisely modulate the wound healing process. Here, we present a pioneering wireless immunomodulatory strategy by integrating amino-modified barium titanate (BTN) nanoparticles with a natural collagen matrix, using oxidized gellan gum (OG) as a crosslinker, to fabricate a tilapia collagen (Col)-based biomimetic piezoelectric hydrogel scaffold (Col/OG/BTN). The hydrogel scaffold exhibits skin-like mechanical properties, controlled biodegradability, and ultrasound (US)-activated piezoelectricity, while providing a three-dimensional porous microenvironment for cell migration and signaling. Under US, the hydrogel scaffold reprograms pro-inflammatory M1 macrophages toward pro-healing M2 macrophages by modulating the phosphoinositide 3-kinase (PI3K) /protein kinase B (Akt) and tumor necrosis factor (TNF) signaling pathways, as revealed by transcriptomics. This immunoregulation synergizes with endothelial cell crosstalk to amplify pro-angiogenic factor secretion. Importantly, in vivo application of the Col/OG/BTN hydrogel scaffold significantly reduces inflammation, enhances angiogenesis, promotes collagen deposition, and stimulates hair follicle regeneration, ultimately achieving high-quality wound healing with functional restoration. In conclusion, this study demonstrates a spatiotemporally controllable approach to modulate the immune microenvironment of inflammatory wounds while promoting vascular regeneration, offering a clinically translatable strategy for regenerative medicine.
Statement of significance
Current wound therapies face challenges in dynamically regulating immune responses and angiogenesis. We developed a tilapia collagen-based piezoelectric hydrogel scaffold integrated with oxidized gellan gum and amino-modified barium titanate nanoparticles (Col/OG/BTN hydrogel scaffold). This ultrasound-activated system uniquely reprograms pro-inflammatory macrophages to pro-healing phenotypes via PI3K/Akt and TNF pathways, synergistically enhancing angiogenesis and hair follicle regeneration. The scaffold eliminates implanted electrodes, offering wireless immunomodulation and vascular restoration, enabling high-quality wound healing with functional skin appendage recovery. This work provides a clinically translatable strategy for inflammatory wound repair through bioelectrical signaling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


