基于超高尺度细胞计数的细胞相互作用制图。
IF 32.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
细胞间的相互作用是至关重要的,协调着生物体的发育、组织的稳态和免疫。最近,使用单细胞基因组技术来解剖物理相互作用细胞的强大方法已经开发出来。然而,这些方法的特点是蜂窝吞吐量低,处理时间长,成本高,并且通常限于预定义的蜂窝类型。在这里,我们介绍了相互作用组学,这是一种基于细胞计量的框架,以超高分辨率和规模准确地绘制所有免疫细胞类型的细胞景观和细胞相互作用。我们证明了我们的方法在研究动力学、作用模式和免疫疗法的个性化反应预测以及体内感染后细胞组成和细胞相互作用动力学的全生物体变化方面的实用性。我们的可扩展框架可以应用于现有的细胞计数数据集或纳入新设计的基于细胞计数的研究,以绘制从基础生物学到应用生物医学的广泛应用的细胞相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
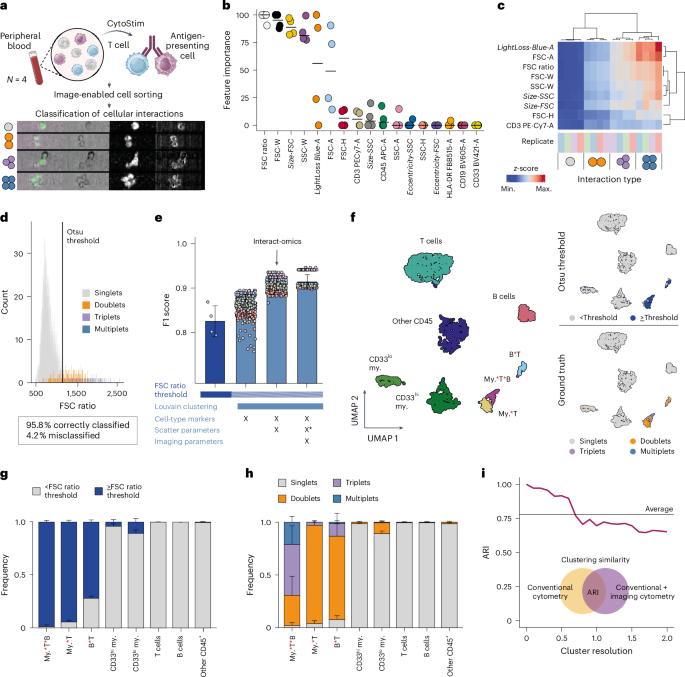
Ultra-high-scale cytometry-based cellular interaction mapping
Cellular interactions are of fundamental importance, orchestrating organismal development, tissue homeostasis and immunity. Recently, powerful methods that use single-cell genomic technologies to dissect physically interacting cells have been developed. However, these approaches are characterized by low cellular throughput, long processing times and high costs and are typically restricted to predefined cell types. Here we introduce Interact-omics, a cytometry-based framework to accurately map cellular landscapes and cellular interactions across all immune cell types at ultra-high resolution and scale. We demonstrate the utility of our approach to study kinetics, mode of action and personalized response prediction of immunotherapies, and organism-wide shifts in cellular composition and cellular interaction dynamics following infection in vivo. Our scalable framework can be applied a posteriori to existing cytometry datasets or incorporated into newly designed cytometry-based studies to map cellular interactions with a broad range of applications from fundamental biology to applied biomedicine. Interact-omics, a high-throughput cytometry-based framework, resolves the cellular interaction landscape.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Methods
生物-生化研究方法
CiteScore
58.70
自引率
1.70%
发文量
326
审稿时长
1 months
期刊介绍:
Nature Methods is a monthly journal that focuses on publishing innovative methods and substantial enhancements to fundamental life sciences research techniques. Geared towards a diverse, interdisciplinary readership of researchers in academia and industry engaged in laboratory work, the journal offers new tools for research and emphasizes the immediate practical significance of the featured work. It publishes primary research papers and reviews recent technical and methodological advancements, with a particular interest in primary methods papers relevant to the biological and biomedical sciences. This includes methods rooted in chemistry with practical applications for studying biological problems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


