芳酮与N,N-二甲基甲酰胺电还原交叉亲电偶联制备α-羟基醛。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
α-羟基醛是一种强大的多功能结构基序,用于生产临床药物和增值化合物。已知的方法通常有局限性,如需要强碱、有毒试剂和需要多步反应。本研究利用N,N-二甲基甲酰胺作为甲酰基化试剂,通过电还原法对芳酮进行非甲基甲酰基化,开发了一种直接合成α-羟醛的方法。这种方法将为α-羟基醛的获取提供一种有效的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
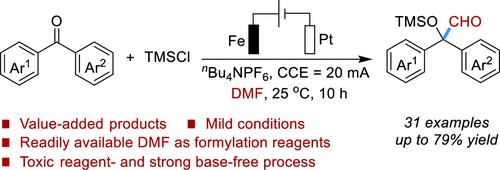
Electroreductive Cross-Electrophile Coupling of Aromatic Ketones and N,N-Dimethylformamide Enabling the Synthesis of α-Hydroxyaldehydes
α-Hydroxyaldehydes are powerful multifunctional structural motifs for the production of clinical drugs and value-added compounds. The known methods typically suffer from limitations such as the requirement for strong bases, toxic reagents, and the necessity of multistep reactions. Here, we developed a straightforward synthetic method of α-hydroxyaldehydes via an electroreductive umpolung formylation of aromatic ketones utilizing readily available N,N-dimethylformamide as formylation reagents. This approach will provide an efficient strategy for the acquisition of α-hydroxyaldehydes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


