栅极电池双电子碱性MnO2转化反应中无序中间体动力学
IF 35.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
除了锂离子电池技术之外,电网规模的存储可能需要广泛的集成。可充电锌-二氧化锰化学具有高可持续性、高安全性和低成本的潜力,使用地球上丰富的基础材料。在碱性电解质中,MnO2阴极可以通过添加含bi的添加剂进行可逆循环,尽管其循环机制尚不清楚。本文介绍了从层状δ-MnO2到Mn(OH)2再到层状δ-MnO2的电化学转化过程中涉及的中间物质。在充电过程中,一种结构类似于层状β-MnOOH的无序中间体在很长一段时间内稳定存在,这与已知Bi具有意想不到的电化学活性的机制相对应。在放电过程中,β-MnOOH仅短暂存在,而非主要物质,说明其循环机制是不对称的。这些发现代表了机械知识的重大进步,可以使工程开发用于商业用途的系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
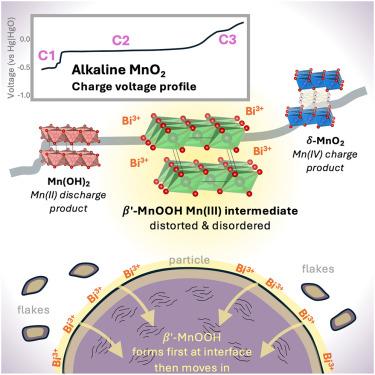
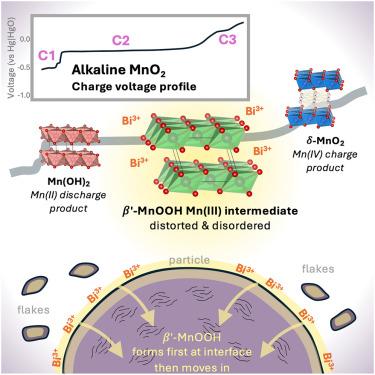
Dynamics of disordered intermediates during the two-electron alkaline MnO2 conversion reaction for grid-scale batteries
Battery technologies beyond Li-ion are likely needed for extensive integration of grid-scale storage. The rechargeable Zn-MnO2 chemistry has the potential for high sustainability, high safety, and low cost, using Earth-abundant basis materials. In an alkaline electrolyte, the MnO2 cathode can cycle reversibly if modified by including a Bi-containing additive, although the cycling mechanism remains mostly unknown. This work presents an account of the intermediate species involved in the electrochemical transformation from layered δ-MnO2 to Mn(OH)2 and back. During charge, a disordered intermediate with a structure resembling layered β-MnOOH exists stably for an extended period, corresponding to a regime known to have unexpected electrochemical activity of Bi. During discharge, β-MnOOH exists only briefly and is never the majority material, revealing that the cycling mechanism is asymmetric. These findings represent a significant advance in mechanistic knowledge and can enable engineering to develop the system for commercial use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


