用胆碱离子液体有机催化亚胺、腈和酰胺的硼氢化反应
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究报道了基于胆碱的离子液体作为有机催化剂在亚胺、腈和酰胺的硼氢化反应中的应用。结果表明,乙酸胆碱具有较强的官能团耐受性,反应条件温和,催化剂负荷低。此外,一锅氢化顺序和偶联反应使腈合成酰胺、亚胺以及伯胺、仲胺和叔胺的产率很高。在这些研究中,通过进行重复的批量实验,证明了催化剂的可回收性。核磁共振研究和DFT计算表明,胆碱和醋酸硼氢化物在这一过程中具有催化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

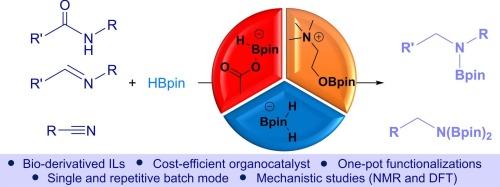
Organocatalytic hydroboration of imines, nitriles, and amides using choline-based ionic liquids
This study reports the application of choline-based ionic liquids as organocatalysts in the hydroboration of imines, nitriles, and amides. The best results characterized by mild reaction conditions and low catalyst loading, were obtained for choline acetate, which showed high functional groups tolerance. Moreover, the one-pot hydroboration sequence and coupling reactions enabled the synthesis of amides, imines, and primary, secondary, and tertiary amines from nitriles with high yields. In these studies, the recyclability of the catalyst was proved by carrying out repetitive batch experiments. The NMR studies and DFT calculations revealed that the choline and acetate borohydrides are catalytically active species in this process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


