金属有机骨架的可控部分自我牺牲促进硝酸盐电还原制氨
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电化学硝酸还原制氨(NO3RR)是最有前途的硝酸盐增值转化途径。然而,NO3RR过程涉及多个电子和质子的转移,因此动力学缓慢,因此迫切需要开发高性能的NO3RR催化剂。在这里,我们制备了超细Cu2O颗粒,并通过控制框架部分的自我牺牲,将其封装在含有配位不饱和Cu2+节点的金属有机框架(mof)中。复合催化剂对NO3RR具有良好的催化性能,NH3产率为6.35 mmol h−1 mgcat−1,法拉第效率为98.6%。密度泛函理论(DFT)计算表明,不饱和Cu2+节点和纳米颗粒之间的协同效应显著降低了所有中间体的势能,从而促进了硝酸盐向氨的有效转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
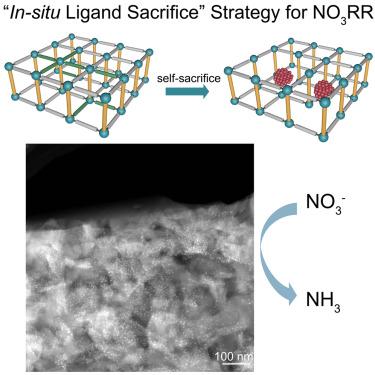
Controllable partial self-sacrifice of metal-organic frameworks for enhancing nitrate electroreduction to ammonia
Electrochemical nitrate reduction to ammonia (NO3RR) is the most promising pathway for the value-added conversion of nitrate. However, the NO3RR process involves the transfer of multi-electrons and protons and hence suffers from slow kinetics, leading to an urgent need to develop high-performance NO3RR catalysts. Here, we prepare ultrafine Cu2O particles in situ generated and encapsulated in metal-organic frameworks (MOFs) containing coordination-unsaturated Cu2+ nodes by the controlled self-sacrifice of a selected part of the framework. The composite catalyst achieves the impressive catalytic performance for NO3RR, with an NH3 yield rate of 6.35 mmol h−1 mgcat−1 and corresponding Faraday efficiency of 98.6%. Density functional theory (DFT) calculations demonstrate that the synergistic effect between unsaturated Cu2+ nodes and nanoparticles markedly decreases the potential energy of all intermediates, thereby facilitating an efficient conversion of nitrate to ammonia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


