聚酰胺基磁性氧化石墨烯纳米复合材料高效去除水溶液中的铅(II)
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
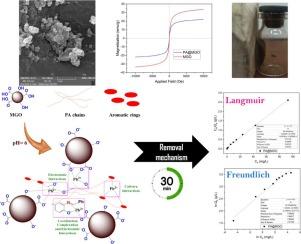
通过直接缩聚反应成功合成了一种含亚胺和偶氮基团的芳香聚酰胺(PA)。采用改进的Hummers法制备氧化石墨烯(GO)。通过原位磁化PA和GO对氧化石墨烯表面进行非共价修饰,制备了新型纳米复合材料PA@MGO。采用不同的分析技术对PA@MGO纳米复合材料的制备进行了评价。结果表明,所制备的纳米复合材料具有球形纳米颗粒,具有较高的饱和磁化强度和热稳定性。该多功能纳米复合材料作为一种新型的纳米吸附剂用于去除水中的铅(II),研究了吸附剂用量、接触时间、溶液pH、Pb(II)初始浓度和选择性等参数。实验结果表明,在相同吸附剂投加量下,PA@MGO的去除率和吸附量分别为99%和36.14 mg/g,高于pH为6时各组分的去除率和吸附量。拟二级动力学和Langmuir等温线模型对PA@MGO吸附Pb(II)提供了较好的解释。此外,选择性结果表明,在Na(I)、K(I)、Mg(II)和Al(III)等竞争离子存在的情况下,Pb(II)的去除过程没有明显影响。所制备的纳米吸附剂能够达到5次循环去除Pb(II),去除效率没有明显下降(10%),并且没有结构变化。在本文中,我们制备了一种新型的热稳定磁性纳米吸附剂,用于选择性去除水中环境中的Pb(II),具有可重复使用长达5次的特性,使其成为一种有潜力的材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Polyamide-based magnetic graphene oxide nanocomposite for efficient Pb(II) removal from aqueous solutions
An aromatic polyamide (PA) containing imine and azo groups was synthesized successfully via a direct polycondensation reaction. Graphene oxide (GO) was prepared by a modified Hummers method. The GO surface was modified non-covalently with PA chains through the in-situ magnetization of PA and GO to prepare the PA@MGO as a novel nanocomposite. The preparation of PA@MGO nanocomposite was evaluated using different analysis techniques. The results showed that the introduced nanocomposite was successfully prepared with sphere-like nanoparticles and exhibited high saturation magnetization, as well as higher thermal stability compared to its components. The multifunctional nanocomposite served as a novel nanosorbent for the removal of Pb(II) from aqueous solutions, and various parameters, such as sorbent dosage, contact time, solution pH, initial concentration of Pb(II), and selectivity, were studied. The experiments showed that the removal efficiency and the adsorption capacity of PA@MGO (99% and 36.14 mg/g, respectively) were higher than those of each component at pH 6 with the same sorbent dosage. The pseudo-second-order kinetics and Langmuir isotherm models provided a proper explanation of the Pb(II) adsorption on PA@MGO. Additionally, the selectivity results showed that the Pb(II) removal process was not significantly affected in the presence of competing ions such as Na(I), K(I), Mg(II), and Al(III). The prepared nanosorbent was capable of up to 5 cycles for Pb(II) removal, without a noticeable decrease in removal efficiency (10%) and no structural changes. In this paper, we prepared a novel thermally stable magnetic nanosorbent for the selective Pb(II) removal from aquatic environments, featuring reusability properties up to five cycles, making it a potential material.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


