乙醇加速4-氨基苯基硼酸氧化法快速、选择性、灵敏地检测青蒿琥酯
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
主要由恶性疟原虫引起的疟疾仍然是一个严重的全球卫生问题,青蒿琥酯因其有效和快速的抗疟疾活性而被广泛使用。然而,青蒿琥酯耐药性的威胁日益严重,需要发展快速、准确和可获得的检测方法。而传统的技术,如高效液相色谱(HPLC)和荧光法提供高灵敏度,他们往往需要复杂的仪器和耗时的程序。相比之下,目前的电化学检测方法可能会遭受高氧化电位或涉及复杂的电极制作。结果本研究提出了一种新的、简单的、高灵敏度的快速检测青蒿琥酯的电化学策略,可在2分钟内获得响应。该方法基于青蒿琥酯在乙醇存在下选择性氧化4-氨基苯基硼酸,其中青蒿琥酯转化为电活性4-氨基苯酚,在约0.083 V下产生明显信号。由于明显的溶剂效应,乙醇的存在显著加快了反应速度,并将分析时间从1小时以上减少到2分钟。该方法线性范围宽(0.5 ~ 135 μM),检出限低(0.12 μM),回收率高(98.70 ~ 103.59%)。该方法具有快速、经济、高效的特点,在制药和临床领域的常规质量控制和现场检测中具有很大的潜力。该研究还为各种电化学应用提供了一种快速加速硼酸脱保护反应的简便方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
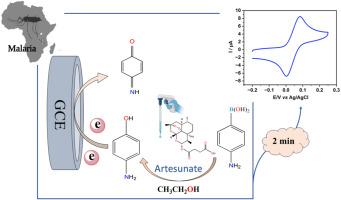
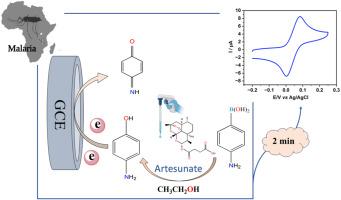
Rapid, selective, and sensitive electrochemical detection of artesunate via 4-aminophenylboronic acid oxidation accelerated by ethanol
Background
Malaria, primarily caused by Plasmodium falciparum, remains a critical global health concern, with artesunate widely administered for its potent and rapid antimalarial activity. However, the growing threat of artesunate resistance necessitates the development of fast, accurate, and accessible detection methods. While conventional techniques such as high-performance liquid chromatography (HPLC) and fluorimetry provide high sensitivity, they often require sophisticated instrumentation and time-consuming procedures. In contrast, current electrochemical detection methods may suffer from high oxidation potentials or involve complex electrode fabrication.
Results
This study presents a new, simple, and highly sensitive electrochemical strategy for the rapid detection of artesunate, achieving a response within 2 min. The method is based on the selective oxidation of 4-aminophenylboronic acid by artesunate in the presence of ethanol, where artesunate is converted into electroactive 4-aminophenol, generating a distinct signal at approximately 0.083 V. The presence of ethanol significantly accelerates the reaction rate and reduces analysis time from more than 1 h to 2 min because of a significant solvent effect. The system demonstrated a wide linear range (0.5–135 μM), a low detection limit of 0.12 μM, and excellent recovery in pharmaceutical formulations (98.70–103.59 %)
Significance
This rapid, cost-effective method holds strong potential for routine quality control and on-site artesunate detection in pharmaceutical and clinical settings. This study also provides a facile way to dramatically accelerate boronate deprotection reactions for various electrochemical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


