全氟化合物对铁还原菌的毒性及其对细胞外电子转移的抑制作用
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
微生物铁还原调节缺氧环境下的生物地球化学循环,但它越来越受到广泛存在的环境污染物的威胁。其中,全氟烷基和多氟烷基物质(PFAS)对铁还原菌的影响往往被忽视。本研究研究了三种PFAS:长链全氟辛酸、短链全氟丁酸和六氟环氧丙烷二聚酸对希瓦氏菌MR-1的毒理学机制,同时描述了它们不同的链长/结构如何破坏细胞外电子转移途径。在20 mg/L时,所有PFAS均表现出浓度依赖性的细菌生长抑制作用,并显著抑制了异化铁还原(dissimilatory iron reduction, DIR)效率。细胞分析显示,这三种PFAS破坏了细胞结构完整性,增加了膜通透性,并引发了氧化损伤。关键的是,PFAS暴露会损害电子传递链组分的生物合成,包括c型细胞色素和核黄素,从而降低电子通量效率并破坏DIR性能。转录组学分析证实了pfas诱导了电子传递链相关基因的下调,并揭示了多层次的毒性机制,包括抑制核糖体生物发生、能量代谢受损和氨基酸转换失调。此外,PFAS混合物对铁还原菌表现出协同毒性,在环境相关浓度下突出了潜在的生态风险。这些发现促进了我们对PFAS生态毒性的理解,并强调了它们可能损害对缺氧生态系统元素循环至关重要的微生物过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
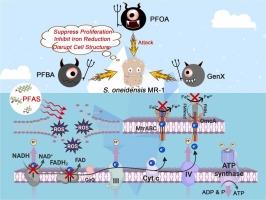
Toxicity of perfluorinated compounds to iron-reducing bacteria and their inhibitory effects on extracellular electron transfer
Microbial iron reduction regulates biogeochemical cycles in anoxic environments, yet it is increasingly threatened by the widespread presence of environmental pollutants. Among these, the impact of per- and polyfluoroalkyl substances (PFAS) on iron-reducing bacteria is often overlooked. This study investigated the toxicological mechanisms of three PFAS: long-chain perfluorooctanoic acid, short-chain perfluorobutanoic acid, and hexafluoropropylene oxide dimer acid, on Shewanella oneidensis MR-1, while delineating how their distinct chain lengths/structures disrupt extracellular electron transfer pathways. At 20 mg/L, all PFAS exhibited concentration-dependent inhibition of bacterial growth and significantly suppressed dissimilatory iron reduction (DIR) efficiency. Cellular analyses revealed all three PFAS undermined cell structural integrity, increased membrane permeability, and triggered oxidative damage. Critically, PFAS exposure impairs the biosynthesis of electron transport chain components, including c-type cytochromes and riboflavin, thereby reducing electron flux efficiency and disrupting DIR performance. Transcriptomic profiling corroborated that PFAS-induced downregulation of electron transport chain-related genes and revealed multi-level toxicity mechanisms, including inhibited ribosome biogenesis, impaired energy metabolism, and dysregulated amino acid conversion. Furthermore, PFAS mixtures exhibited synergistic toxicity to iron-reducing bacteria, highlighting potential ecological risks at environmentally relevant concentrations. These findings advance our understanding of PFAS ecotoxicity and highlight their potential to impair microbial processes critical to elemental cycling in anoxic ecosystems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


