胰腺癌免疫治疗的经典与创新策略
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
胰腺导管腺癌(PDAC)是一种预后不佳的侵袭性恶性肿瘤。免疫检查点抑制剂(ICIs)的免疫治疗,无论是单独治疗,与其他ICIs联合治疗,还是与化疗联合治疗,都能显著改善几种实体瘤的预后。然而,由于多种耐药机制,其在PDAC中的疗效仍然有限。PDAC免疫治疗耐药的关键决定因素包括阻碍免疫细胞浸润的物理屏障,如异常的脉管系统、癌症相关成纤维细胞(CAFs)和肿瘤微环境(TME)中过多的透明质酸沉积。此外,PDAC的特点是免疫抑制性TME富集了调节性T细胞(Tregs)和髓源性抑制细胞(MDSCs),并且由于KRAS突变、MYC过表达和低肿瘤突变负担,肿瘤细胞的免疫原性较低,进一步损害抗肿瘤免疫。这篇综述讨论了先进的药物输送系统,以克服免疫治疗耐药的决定因素并改善结果,探讨了新兴的免疫治疗策略,包括过继细胞疗法,癌症疫苗,以及通过粪便微生物群移植或肿瘤内细菌接种作为TME调节剂的微生物群的潜在作用。鉴于微生物群在PDAC中的矛盾作用,需要明确定义有利菌株及其选择。还探讨了涉及工程细菌和人工智能应用的新兴方法。最后,我们提出了一种创新的多模式免疫治疗方法的假设概念框架,以克服PDAC的耐药和改善临床结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
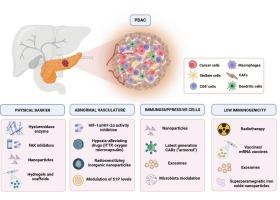
Classic versus innovative strategies for immuno-therapy in pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy with a dismal prognosis. Immunotherapy with immune checkpoint inhibitors (ICIs), either as monotherapy, in combination with other ICIs, or alongside chemotherapy, has significantly improved outcomes in several solid tumors. However, its efficacy in PDAC remains limited due to multiple resistance mechanisms.
Key determinants of immunotherapy resistance in PDAC include physical barriers that hinder immune cells infiltration, such as aberrant vasculature, cancer-associated fibroblasts (CAFs), and excessive hyaluronic acid deposition in the tumor microenvironment (TME). Additionally, PDAC is characterized by an immunosuppressive TME enriched with regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and by low immunogenicity of tumor cells due to KRAS mutations, MYC overexpression, and a low tumor mutational burden, further impairing antitumor immunity.
This review discusses advanced drug delivery systems to overcome determinants of immunotherapy resistance and to improve outcomes, explores emerging immunotherapy strategies, including adoptive cell therapies, cancer vaccines, and the potential role of microbiota as modulator of TME through fecal microbiota transplantation or intratumoral bacterial inoculation. Given the ambivalent role of microbiota in PDAC, the need for a clear definition of favorable strains and their selection is highlighted. Emerging approaches involving engineered bacteria and artificial intelligence applications are also explored.
Finally, we propose a hypothetical conceptual framework for an innovative multimodal immunotherapy approach to overcome resistance and improve clinical outcomes in PDAC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


