生物原料药冷冻和解冻的冷冻浓缩模型和实验测量。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-08-05
DOI:10.1016/j.ejpb.2025.114812
引用次数: 0
摘要
冻融过程是决定原料药和生物原料药冷冻浓度分布的关键因素,对蛋白质的稳定性有着直接而不可避免的影响。由于冷冻浓缩的关键影响——取决于其分布,是稳定蛋白质还是破坏蛋白质的稳定——了解冷冻和解冻过程中发生的具体机制和变化对于确保BDS的稳定性以及延伸到药品(DP)的稳定性至关重要。这项工作提出了在一个两升的塑料容器中由冷冻和解冻引起的蛋白质冷冻浓缩的计算模型。本研究利用固定网格模型和物种分离模型,模拟原料药的典型加工过程,研究了导致容器内蛋白质浓度分布变化的冷冻和解冻动力学。该模型采用了一个分配系数来表示BDS溶液阶段和冷冻冰阶段之间的蛋白质分布。在冰形成过程中溶质(如蛋白质)的分配,以及富含蛋白质的液体从冰-液界面向容器底部的重力沉降,在超低温冰箱中冷冻时产生了低温浓缩。对先前冷冻的溶液进行+23°C水浴解冻的模拟显示,由于在解冻过程中建立的自由对流无法克服基于密度的分离,因此容器底部的蛋白质浓度进一步增加。模拟结果与2L瓶中60 mg/mL人血清白蛋白溶液冷冻和解冻的实验测量结果一致,显示了利用计算技术设计工艺和设备的希望,这些工艺和设备可以减少冻融操作过程中蛋白质的冷冻浓度和分离梯度,从而提高药品生产和产品质量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
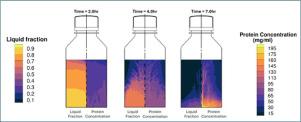
Cryoconcentration modeling and experimental measurements for freezing and thawing of a biologic bulk drug substance
The freezing and thawing process is a key determinant of the cryoconcentration distribution in pharmaceutical and biological bulk drug substances (BDS), which has a direct and inevitable impact on protein stability. Due to the critical influence of cryoconcentration — either stabilizing or destabilizing the protein depending on its distribution — understanding the specific mechanisms and changes occurring during freezing and thawing is essential for ensuring the stability of the BDS and, by extension, the drug product (DP). This work presents computational modeling of protein cryoconcentration induced by freezing and thawing in a two-liter plastic container. The study utilized fixed-grid modeling with a species segregation model to investigate freezing and thawing dynamics that lead to a shift in protein concentration distribution inside the container, simulating typical processing for pharmaceutical bulk drug substance. The model incorporates a partition coefficient to represent protein distribution between the BDS solution phase and the frozen ice phase. The solute (such as protein) partitioning during ice formation and gravity settlement of protein-rich liquid from the ice–liquid interface towards the bottom of a container creates cryoconcentration during freezing in an ultra-low temperature freezer. Simulations of previously frozen solution subjected to water-bath thawing at showed a further increase of protein concentration at the bottom of the container due to the inability of the free convective currents set up during the thawing process to overcome the density based segregation. The modeling results were consistent with experimental measurements of freezing and thawing of Human serum albumin solution at 60 mg/mL in 2 L bottles, showing promise for the utilization of computational techniques to design process and equipment that reduces protein cryoconcentration and segregation gradients during freeze–thaw operation improving pharmaceutical manufacturing and product quality.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


