高价碘试剂促进吲哚衍生物烷氧基化:3,3 -二取代吲哚的合成
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了吲哚与高价碘基硝基氧化试剂的无催化剂分子间脱芳反应。在温和的条件下,得到了各种带有季碳立体中心的烷氧化3,3 -二取代氧吲哚,收率可达92%。同时,得到的产物可以顺利进行多种转化,包括Sonogashira偶联反应、Suzuki偶联反应和BBr3介导的去甲基化反应。此外,以该方法为关键步骤合成了天然产物(±)- convolutamydine A,显示了该方法的合成潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
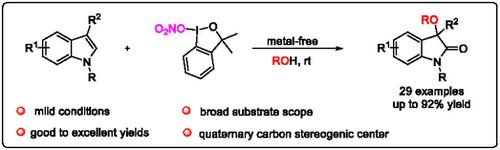
Hypervalent Iodine Reagent‐Promoted Alkoxylation of Indole Derivatives: Synthesis of 3,3‐Disubstituted Oxindoles
A catalyst‐free intermolecular dearomatization reaction of indoles with hypervalent‐iodine‐based nitrooxylating reagent is reported. Various alkoxyated 3,3‐disubstituted oxindoles bearing a quaternary carbon stereogenic center are obtained in good to excellent yields (up to 92%) under mild conditions. Meanwhile, the obtained products can undergo a variety of transformations smoothly, including Sonogashira coupling reaction, Suzuki coupling reaction, and demethylation reaction mediated by BBr3. In addition, natural product (±)‐convolutamydine A is synthesized by employing this method as the key step, showcasing the synthetic potential of the current method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


