50年的单克隆:抗体治疗的过去、现在和未来
IF 60.9
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
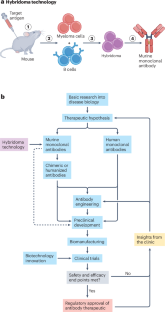
1975年,Köhler和Milstein发明了杂交瘤技术,用于产生具有预定抗原结合特异性的小鼠单克隆抗体。单克隆抗体作为生物医学研究试剂的广泛使用,以及迄今为止全球至少批准了212种抗体治疗方法,治疗了数千万患者,证明了单克隆抗体的变革性影响。抗体技术的进步,如人源化和人类抗体生成的稳健方法,减轻了小鼠抗体作为治疗手段的主要局限性。这些技术与生物制造的进步相结合,帮助开启了抗体治疗的现代时代。除了IgG,抗体疗法已经发展成多种形式,包括双特异性抗体和抗体-药物偶联物。此外,抗体片段已被开发为独立治疗和靶向细胞治疗,特别是嵌合抗原受体T细胞。抗体技术的这些进步,加上能够皮下递送的创新,提高了许多患者的治疗效果和抗体治疗的便利性。针对人类表皮生长因子受体2 (HER2)阳性癌症的多代抗体疗法和针对血液学癌症和免疫疾病的B细胞靶向疗法说明了这一概念。最后,我们简要地展望了抗体治疗的未来发展方向,包括人工智能在抗体鉴定和多参数优化方面的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Fifty years of monoclonals: the past, present and future of antibody therapeutics
In 1975, Köhler and Milstein invented hybridoma technology for the generation of murine monoclonal antibodies with predetermined antigen-binding specificity. The transformative impact of monoclonal antibodies is demonstrated by their ubiquitous use as biomedical research reagents and the worldwide approval of at least 212 antibody therapeutics with tens of millions of patients treated to date. Advances in antibody technologies, such as humanization and robust methods for human antibody generation, mitigated the major limitations of murine antibodies as therapeutics. These technologies, combined with progress in biomanufacturing, helped to launch this modern era of antibody therapeutics. Beyond IgG, antibody therapeutics have blossomed into multiple alternative formats, including bispecific antibodies and antibody–drug conjugates. Additionally, antibody fragments have been developed as stand-alone therapeutics and to target cell therapies, notably chimeric antigen receptor T cells. These advances in antibody technologies, plus innovation enabling subcutaneous delivery, have improved the therapeutic benefits and convenience of antibody treatment for many patients. This concept is illustrated here by multiple generations of antibody therapeutics for human epidermal growth factor receptor 2 (HER2)+ cancers and B cell-targeted therapies for haematological cancers and immunological diseases. Finally, we opine briefly on some of the many promising future directions with antibody therapeutics, including the application of artificial intelligence for antibody identification and multi-parameter optimization. Fifty years ago, Köhler and Milstein introduced the world to hybridoma technology for the generation of monoclonal antibodies. Scientists have subsequently built upon this seminal discovery to develop antibody-based therapies for numerous diseases, with millions of patients benefiting from such drugs. To mark 50 years of monoclonal antibodies, this Review from Chan, Martyn and Carter provides an overview of how antibody engineering strategies have continued to improve antibody-based therapeutics, chiefly focusing on antibody-mediated targeting of B cells and also human epidermal growth factor receptor 2 (HER2)+ cancers. The authors also highlight the promise of emerging tools, including artificial intelligence, for development of the next generation of antibody-based therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Immunology
医学-免疫学
CiteScore
93.40
自引率
0.40%
发文量
131
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Immunology is a journal that provides comprehensive coverage of all areas of immunology, including fundamental mechanisms and applied aspects. It has two international standard serial numbers (ISSN): 1474-1733 for print and 1474-1741 for online. In addition to review articles, the journal also features recent developments and new primary papers in the field, as well as reflections on influential people, papers, and events in the development of immunology. The subjects covered by Nature Reviews Immunology include allergy and asthma, autoimmunity, antigen processing and presentation, apoptosis and cell death, chemokines and chemokine receptors, cytokines and cytokine receptors, development and function of cells of the immune system, haematopoiesis, infection and immunity, immunotherapy, innate immunity, mucosal immunology and the microbiota, regulation of the immune response, signalling in the immune system, transplantation, tumour immunology and immunotherapy, and vaccine development.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


