在体外和体内,RENBP抑制增强了抗原呈递细胞的代谢聚糖标记效率
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
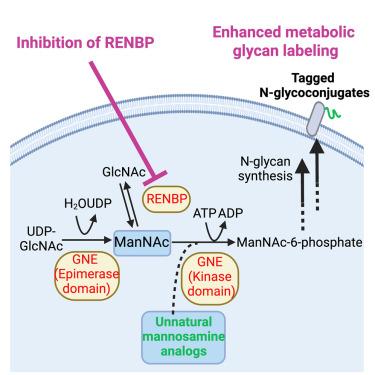
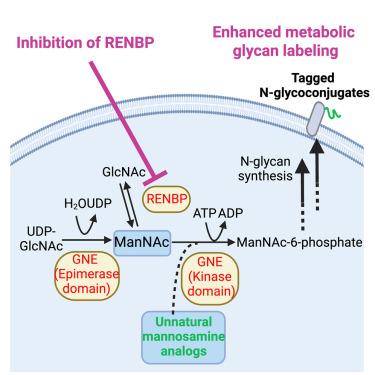
非天然糖的代谢糖工程为在细胞膜上引入独特的化学标签以进行后续的偶联提供了有力的工具。然而,作为适应性免疫的关键介质,抗原呈递细胞(antigen-presenting cells, APCs)的代谢聚糖标记效率往往较低。在这里,我们报道了apc上调GlcNAc 2- epimase (RENBP),并且RENBP抑制导致apc(包括树突状细胞(1.2倍)、巨噬细胞(1.3倍)和B细胞(1.4倍)中四乙酰基- n -叠氮乙酰甘油三胺(AAM)的标记效率提高。RENBP抑制可以优先提高AAM在APCs中的标记效率,并选择性地提高AAM对叠氮半乳糖胺的标记效率。我们进一步证明,RENBP抑制剂可以在体内改善aam介导的B细胞和其他apc的标记,对B细胞的增强效果最大(3倍),持续7天。我们的研究揭示了一种简单的方法来改善apc的代谢聚糖标记,使apc靶向免疫疗法的发展成为可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
RENBP inhibition amplifies metabolic glycan labeling efficiency of antigen-presenting cells in vitro and in vivo
Metabolic glycoengineering of unnatural sugars provides a powerful tool to introduce unique chemical tags onto cell membrane for subsequent conjugation of cargos. However, the metabolic glycan labeling efficiency of antigen-presenting cells (APCs), the key mediators of adaptive immunity, is often low. Here, we report that APCs upregulate GlcNAc 2-epimerase (RENBP) and that RENBP inhibition leads to improved labeling efficiency of tetraacetyl-N-azidoacetylmannosamine (AAM) in APCs, including dendritic cells (1.2-fold), macrophages (1.3-fold), and B cells (1.4-fold) in vitro. RENBP inhibition can preferentially enhance AAM labeling efficiency in APCs than in non-APCs and selectively enhance the labeling efficiency of AAM over azido-galactosamine. We further demonstrate that RENBP inhibitors can improve AAM-mediated labeling of B cells and other APCs in vivo, with the largest enhancement for B cells (>3-fold) for 7 days. Our study uncovers a facile approach to improving metabolic glycan labeling of APCs, enabling the development of APC-targeted immunotherapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


