用未成熟的ESCs和iPSCs制备小鼠原肠胚后全胚胎模型
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
完全由原始胚胎干细胞(nESCs)产生的原肠胚后干细胞衍生的小鼠胚胎模型(SEMs)强调了它们产生胚胎和胚胎外谱系的能力。然而,现有的小鼠SEMs方案依赖于胚胎外谱系的单独诱导和转录因子的异位表达来诱导nESC分化为滋养外胚层(TE)或原始内胚层(PrE)。在这里,我们证明了小鼠nESCs和初代诱导多能干细胞(niPSCs)可以通过信号通路调节同时共诱导产生PrE和TE胚外细胞,这些细胞可以自组织成胚日(E) 8.5-E8.75无转基因(TF)的SEMs。我们还设计了一种替代条件(AC)初始培养基,该培养基在体外稳定tf - sem诱导的OCT4+/NANOG+ nESC集落,这些集落共同表达拮抗CDX2和/或GATA6胚外命运主调控因子,并在保持TE和PrE分化的同时自我更新。这些发现改善了小鼠的SEM策略,并阐明了在体外扩增小鼠原始多能细胞固有的和休眠的胚胎外可塑性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
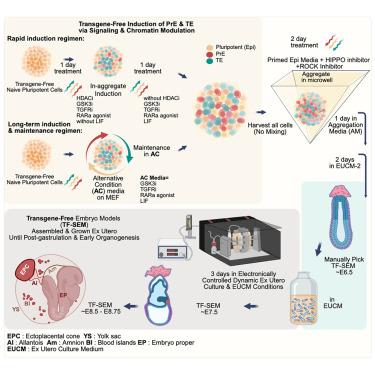
Transgene-free generation of mouse post-gastrulation whole embryo models solely from naive ESCs and iPSCs
The generation of post-gastrulation stem cell-derived mouse embryo models (SEMs) exclusively from naive embryonic stem cells (nESCs) has underscored their ability to give rise to embryonic and extra-embryonic lineages. However, existing protocols for mouse SEMs rely on the separate induction of extra-embryonic lineages and on ectopic expression of transcription factors to induce nESC differentiation into trophectoderm (TE) or primitive endoderm (PrE). Here, we demonstrate that mouse nESCs and naive induced pluripotent stem cells (niPSCs) can be simultaneously co-induced, via signaling pathway modulation, to generate PrE and TE extra-embryonic cells that self-organize into embryonic day (E) 8.5–E8.75 transgene-free (TF) SEMs. We also devised an alternative condition (AC) naive media that in vitro stabilizes TF-SEM-competent OCT4+/NANOG+ nESC colonies that co-express antagonistic CDX2 and/or GATA6 extra-embryonic fate master regulators and self-renew while remaining poised for TE and PrE differentiation, respectively. These findings improve mouse SEM strategies and shed light on amplifying an inherent and dormant extra-embryonic plasticity of mouse naive pluripotent cells in vitro.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


