HNF1A和A1CF协调β细胞转录剪接轴,该轴在2型糖尿病中被破坏
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
2型糖尿病(T2D)是一种以胰腺β细胞功能障碍和胰岛素抵抗为特征的破坏性慢性疾病,其病理生理机制尚不清楚。HNF1A编码转录因子肝细胞核因子-1 α,是孟德尔型糖尿病中最常见的突变基因。HNF1A也携带功能缺失或功能获得的编码变体,分别易患或预防多基因T2D。然而,hnf1a缺陷型糖尿病的发病机制尚不清楚。我们现在证明糖尿病是由β细胞自主缺陷引起的,并确定了HNF1A的直接β细胞基因组靶点。这揭示了一个调控轴,HNF1A控制A1CF的转录,A1CF协调RNA剪接程序,包括调节β细胞功能的基因。这种HNF1A-A1CF转录剪接轴在T2D个体的β细胞中被抑制,而减少胰岛A1CF的遗传变异与血糖升高和T2D易感性相关。因此,我们的研究结果确定了一个线性层次结构,该结构协调β细胞特异性转录和剪接程序,并将该途径与T2D发病机制联系起来。本文章由计算机程序翻译,如有差异,请以英文原文为准。
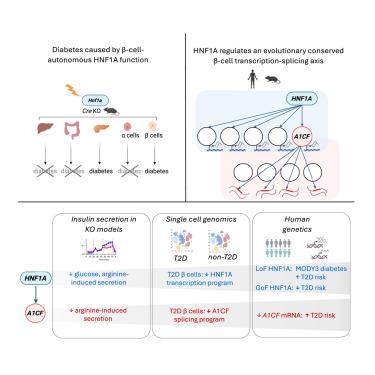
HNF1A and A1CF coordinate a beta cell transcription-splicing axis that is disrupted in type 2 diabetes
Type 2 diabetes (T2D) is a devastating chronic disease marked by pancreatic β cell dysfunction and insulin resistance, whose pathophysiology remains poorly understood. HNF1A, which encodes transcription factor hepatocyte nuclear factor-1 alpha, is the most commonly mutated gene in Mendelian diabetes. HNF1A also carries loss- or gain-of-function coding variants that respectively predispose to or protect against polygenic T2D. The mechanisms underlying HNF1A-deficient diabetes, however, are still unclear. We now demonstrate that diabetes arises from β cell-autonomous defects and identify direct β cell genomic targets of HNF1A. This uncovered a regulatory axis where HNF1A controls transcription of A1CF, which orchestrates an RNA splicing program encompassing genes that regulate β cell function. This HNF1A-A1CF transcription-splicing axis is suppressed in β cells from T2D individuals, while genetic variants reducing pancreatic islet A1CF are associated with increased glycemia and T2D susceptibility. Our findings, therefore, identify a linear hierarchy that coordinates β cell-specific transcription and splicing programs and link this pathway to T2D pathogenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


