去甲thindrone会引起氧化应激,肠道损伤,并扰乱成年雌性西蚊鱼(Gambusia affinis)肠道微生物群组成。
IF 4.3
3区 环境科学与生态学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology C-toxicology & Pharmacology
Pub Date : 2025-08-05
DOI:10.1016/j.cbpc.2025.110305
引用次数: 0
摘要
Norethindrone (NET)是一种广泛使用的合成黄体酮,在多种水生生态系统中持续被检测到,引起了人们对其潜在生态风险的严重关注。本研究采用环境相关浓度NET(5.0、50.0和500.0 ng/L)对成年雌性西蚊鱼(Gambusia affinis)暴露42 天,评价其对胃肠道的毒性。结果表明,净净暴露显著诱导肠道损伤(即肠道绒毛间隙增加,柱状上皮细胞高度降低,肠肌层厚度降低,杯状细胞密度降低)。这些病理改变可能最终导致G. affinis显著的生长抑制。此外,抗氧化生理发生显著变化,包括活性氧(ROS)、丙二醛(MDA)、超氧化物歧化酶(SOD)、谷胱甘肽s -转移酶(GST)和谷胱甘肽过氧化物酶(GPX)水平升高。转录组学和蛋白-蛋白相互作用(PPI)分析表明,净暴露诱导氧化应激相关通路的激活,特别是铁死亡和PI3K/AKT信号通路。最后,16S rRNA测序分析显示,NET暴露显著改变了胃肠道内微生物群落的结构和功能。总之,本研究阐明了净净诱导氧化应激和肠道组织损伤的机制,强调了净净暴露对水生生物的健康影响,并为其生态毒理学风险提供了重要见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
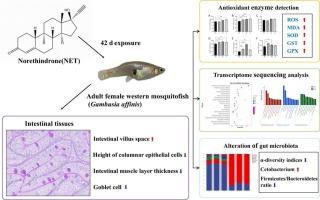
Norethindrone causes oxidative stress, intestinal injury, and disturbed gut microbiota composition in adult female western mosquitofish (Gambusia affinis)
Norethindrone (NET), a widely used synthetic progestin, has been consistently detected in diverse aquatic ecosystems, raising significant concerns about its potential ecological risks. In this study, adult female western mosquitofish (Gambusia affinis) were exposed to environmentally relevant concentrations of NET (5.0, 50.0, and 500.0 ng/L) for 42 days to evaluate the toxicity on gastrointestinal (GI) tract. The results demonstrated that NET exposure significantly induced intestinal damage (i.e., increased villus space in the gut, reduced height of columnar epithelial cells, decreased intestinal muscle layer thickness, and diminished goblet cell density). These pathological alterations might ultimately lead to significant growth inhibition in G. affinis. Furthermore, significant alterations in the antioxidant physiology were observed, including elevated levels of reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione S-transferase (GST), and glutathione peroxidase (GPX). Transcriptomic and protein-protein interaction (PPI) analyses demonstrated that NET exposure induced the activation of oxidative stress-associated pathways, particularly the ferroptosis and PI3K/AKT signaling pathways. Finally, 16S rRNA sequencing analysis revealed that NET exposure significantly altered the structure and function of the microbial community within the GI tract. In conclusion, this study clarifies the mechanisms of NET induced oxidative stress and intestinal tissue damage, highlights the health implications of NET exposure in aquatic organisms, and provides critical insights into its ecotoxicological risks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.50
自引率
5.10%
发文量
206
审稿时长
30 days
期刊介绍:
Part C: Toxicology and Pharmacology. This journal is concerned with chemical and drug action at different levels of organization, biotransformation of xenobiotics, mechanisms of toxicity, including reactive oxygen species and carcinogenesis, endocrine disruptors, natural products chemistry, and signal transduction with a molecular approach to these fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


